Preparation of boron nitride material anchored cobalt ferrite composite catalyst and application of boron nitride material anchored cobalt ferrite composite catalyst in catalytic degradation of oxytetracycline
A composite catalyst, boron nitride technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve the loss of active sites, limit the scope of use, It is easy to agglomerate and accumulate and other problems, and achieve the effect of efficient fixation, excellent degradation performance and good cycle stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
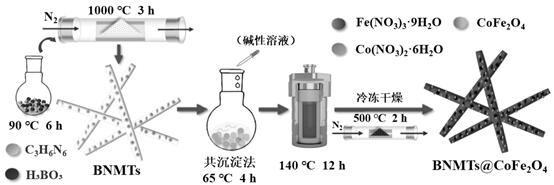
[0094] (1) Preparation of BNMTs:
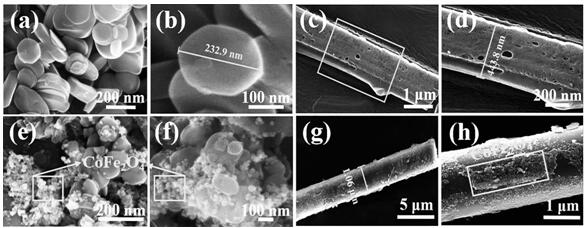
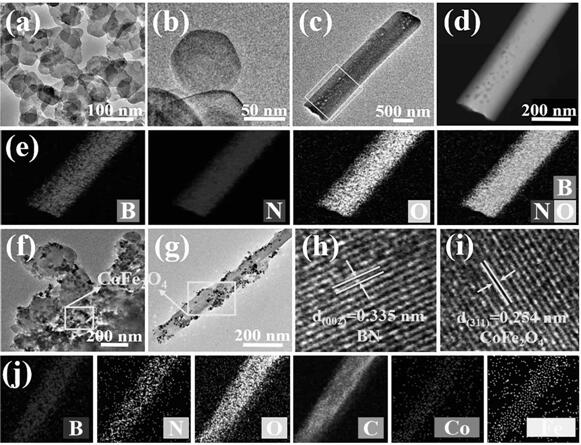
[0095] H 3 BO 3 (2 mmol) and C 3 h 6 N 6 (1 mmol) dissolved in deionized water. Heated to 90°C in an oil bath and assisted stirring for 6 h to form a colorless and transparent solution. It was cooled to room temperature, and after the white precipitate was precipitated, it was suction filtered and dried. The tube furnace was repeatedly evacuated, and then the above white precipitate was placed in N 2 Heating to 1000 °C under atmosphere and calcining for 3 h, the white powdery product obtained is BNMTs.
[0096] (2) BNMTs@CoFe 2 o 4 Preparation of:
[0097] Add 0.5 g BNMTs, 2 mmol Co(NO 3 ) 2 ·6H 2 O and 1 mmol Fe (NO 3 ) 3 9H 2 O stirred for 30min to make it evenly dispersed. with NaOH and Na 2 CO 3 The mixed solution adjusts the pH of the above solution at 10 to 10.5. It was sonicated for 30 min and then stirred at 65°C for 4 h, then transferred to an autoclave while hot and reacted at 140°C for 12 h. Then the precipitat...
Embodiment 2
[0098] Example 2 H-BN@CoFe 2 o 4 preparation of
[0099] Add 0.5 g H-BN, 2 mmol Co(NO 3 ) 2 ·6H 2 O and 1 mmol Fe (NO 3 ) 3 9H 2 O stirred for 30min to make it evenly dispersed. with NaOH and Na 2 CO 3 The mixed solution adjusts the pH of the above solution at 10 to 10.5. It was sonicated for 30 min and then stirred at 65°C for 4 h, then transferred to an autoclave while hot and reacted at 140°C for 12 h. Then the precipitate was repeatedly washed with ethanol and deionized water, and freeze-dried at -40 °C for 24 h. The obtained product was H-BN@CFLDH. Put H-BN@CFLDH in N 2 Calcined in a tube furnace at 500 °C for 2 h under protection to obtain H-BN@CoFe 2 o 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com