Preparation method of high-purity surgical grade alpha-calcium sulfate hemihydrate with adjustable crystal size
A technology of calcium sulfate hemihydrate, crystal size, applied in chemical instruments and methods, calcium/strontium/barium sulfate, prostheses, etc., can solve the problem of unfavorable mass production, limited ability to prepare products, calcium sulfate hemihydrate crystal size Uncontrollable and other problems, to achieve the effect of high-efficiency process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
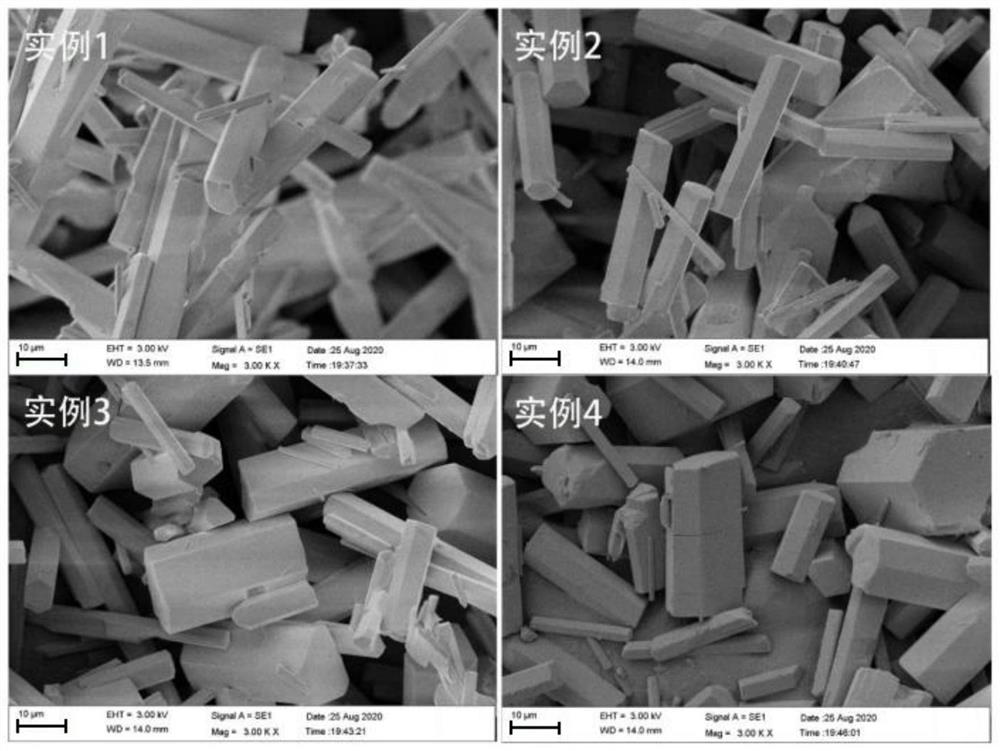
Image
Examples
Embodiment 1
[0031] A kind of high-purity crystal size is the preparation method of α-calcium sulfate hemihydrate of 40-50m, and the steps are as follows:
[0032] Weighed 0.1 g of citric acid and 0.4 g of aluminum sulfate and placed them in a 250 mL beaker, and added 150 mL of deionized water into the beaker, and ultrasonically dissolved them for 2 minutes to obtain a reaction solvent solution. Weigh 50g of calcium sulfate dihydrate and place it in a beaker, and stir it mechanically to make it evenly mixed.
[0033] The beaker was placed in an autoclave, the reaction temperature was 120° C., the reaction pressure was 0.11 MPa, and the reaction time was 2 hours.
[0034] After the reaction, the crude product was taken out, filtered and washed 5 times with 80% alcohol to obtain the product.
[0035] The product was dried in a vacuum oven at 60°C for 3 hours, and then vacuum-packed after drying.
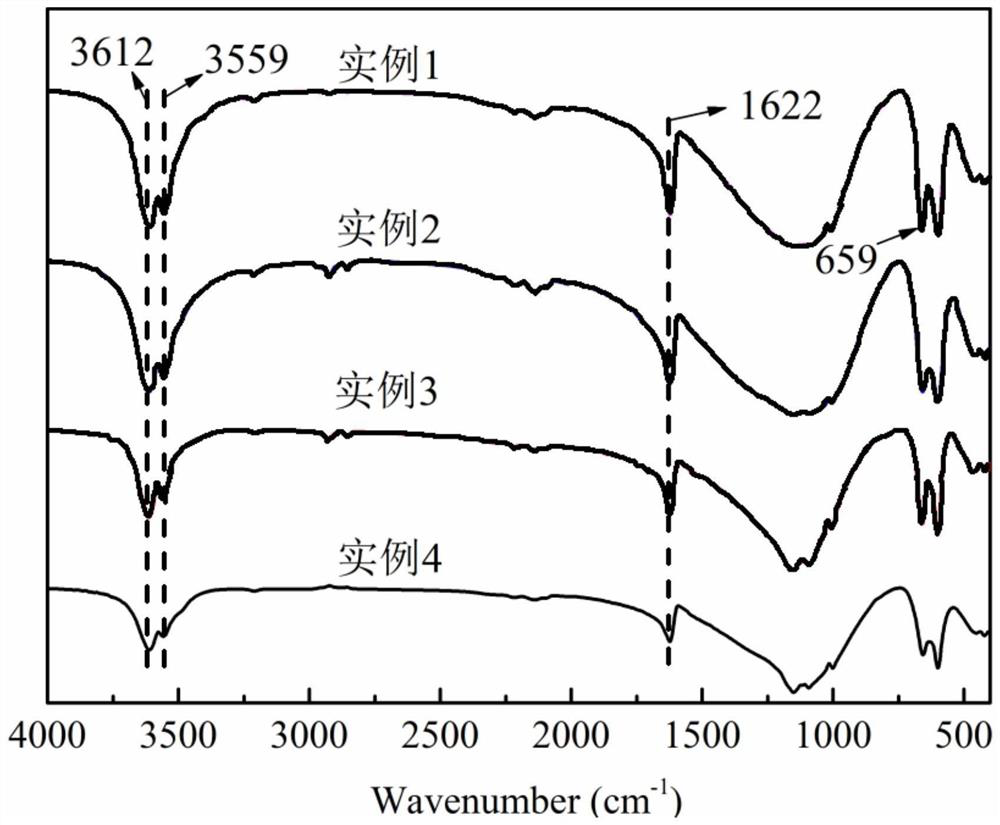
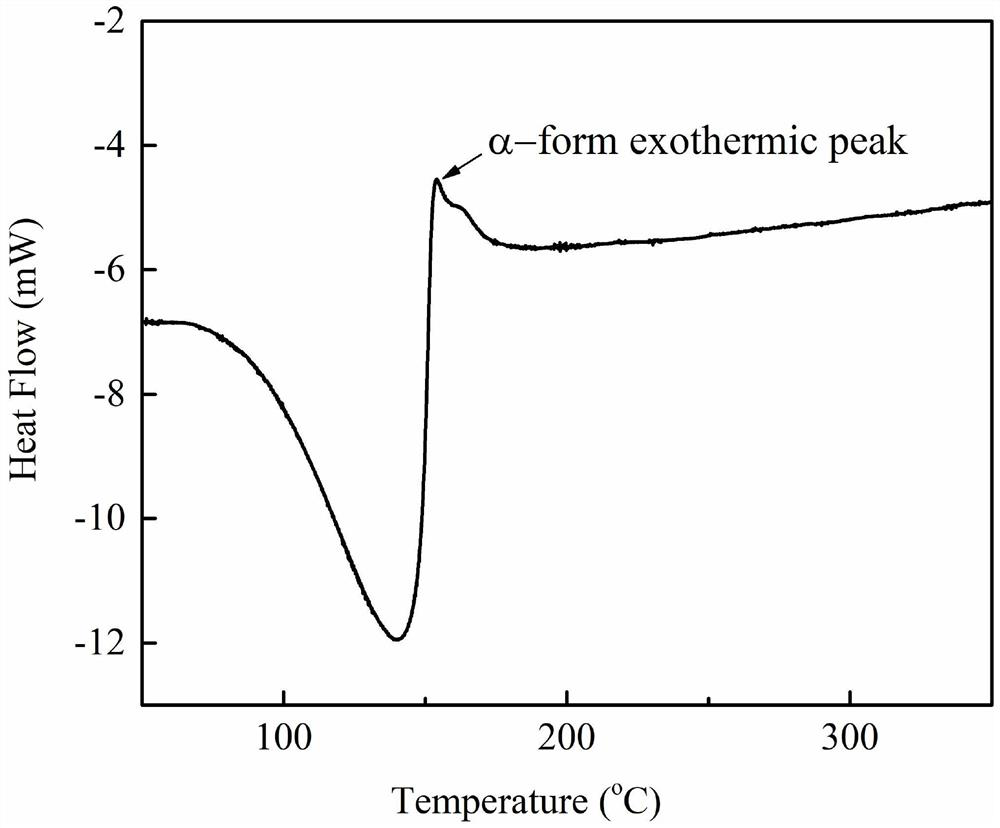
[0036] Performance Characterization:
[0037] The α-calcium sulfate hemihydrate prepared in ...
Embodiment 2
[0044] In the present embodiment, a kind of high-purity crystal size is the preparation method of α-calcium sulfate hemihydrate of 30-40m, and the steps are as follows:
[0045] Weigh 0.1 g of citric acid and 0.3 g of aluminum sulfate and place them in a 250 mL beaker, and add 100 mL of deionized water into the beaker, and ultrasonically dissolve for 3 minutes to obtain a reaction solvent. Weigh 50g of calcium sulfate dihydrate and place it in a beaker, and stir it mechanically to make it evenly mixed.
[0046] The beaker was placed in an autoclave, the reaction temperature was 125° C., the reaction pressure was 0.14 MPa, and the reaction time was 1.5 h.
[0047] After the reaction, the crude product was taken out, filtered and washed 5 times with 85% alcohol to obtain the product.
[0048] The product was dried in a vacuum oven at 60°C for 2.5 hours, and then vacuum-packed after drying.
[0049] Performance Characterization:
[0050] The α-calcium sulfate hemihydrate prepa...
Embodiment 3
[0055] In the present embodiment, a kind of high-purity crystal size is the preparation method of α-calcium sulfate hemihydrate of 20-30m, and the steps are as follows:
[0056] Weigh 0.05g of citric acid and 0.035g of aluminum sulfate respectively and place them in a 250mL beaker, and add 50mL of deionized water into the beaker, and ultrasonically dissolve for 4min to obtain a reaction solvent. Weigh 50g of calcium sulfate dihydrate and place it in a beaker, and stir it mechanically to make it evenly mixed.
[0057] The beaker was placed in an autoclave, the reaction temperature was 130° C., the reaction pressure was 0.18 MPa, and the reaction time was 1.5 h.
[0058] After the reaction, the crude product was taken out, filtered and washed 5 times with 90% alcohol to obtain the product.
[0059] The product was dried in a vacuum oven at 60°C for 2 hours, and then vacuum-packed after drying.
[0060] Performance Characterization:
[0061] The α-calcium sulfate hemihydrate p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| compressive strength | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com