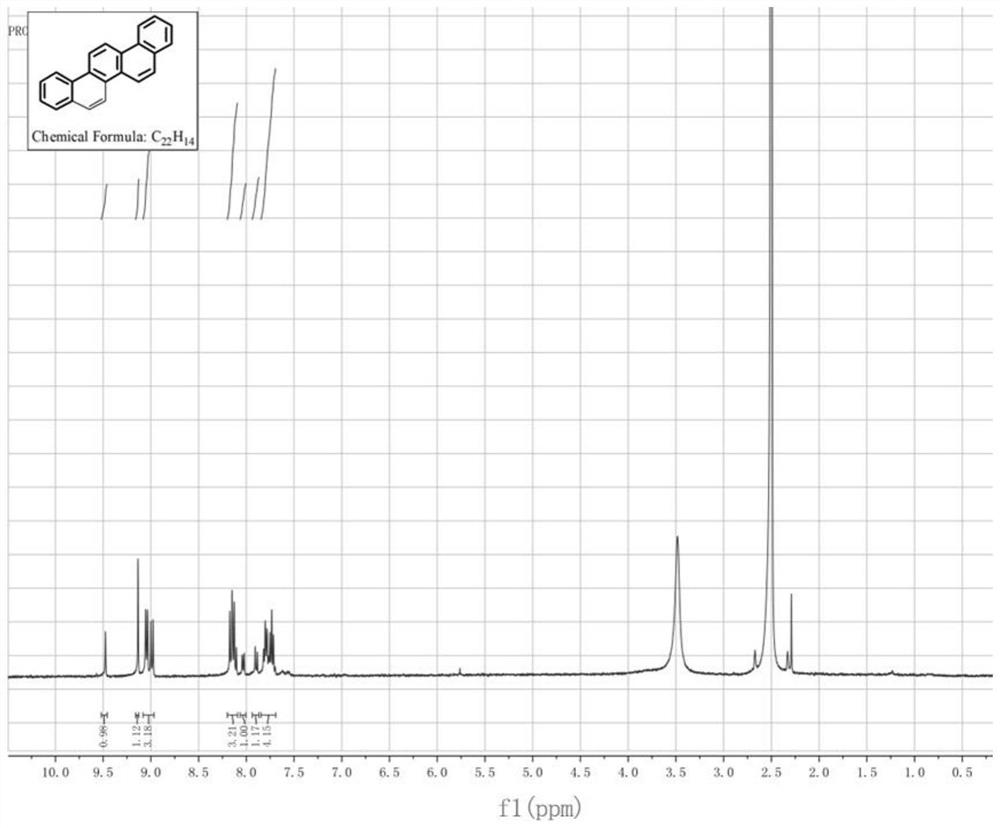
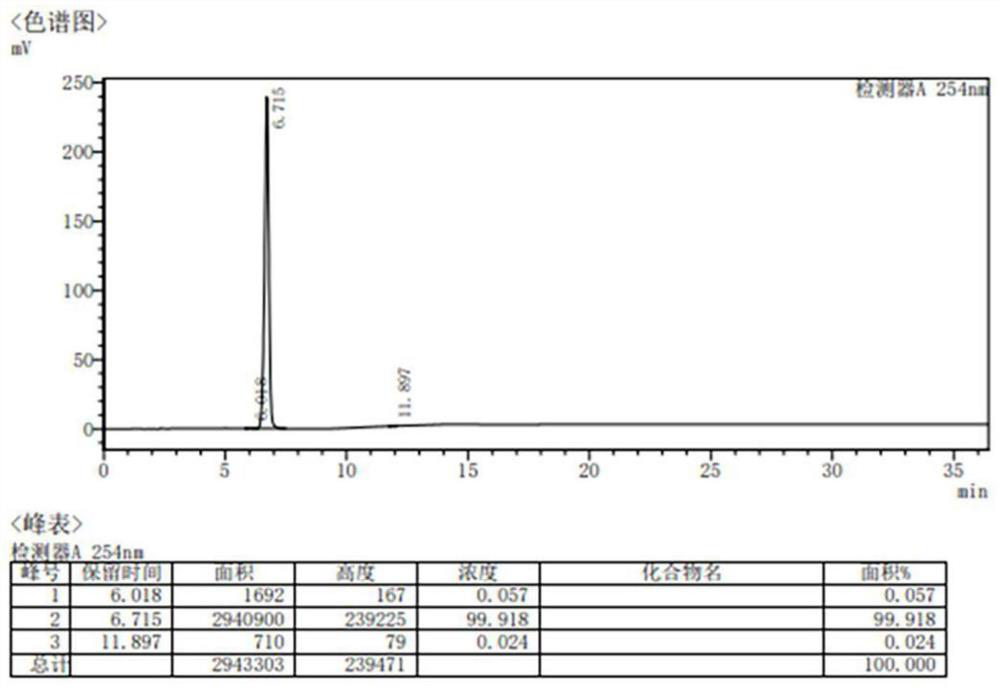
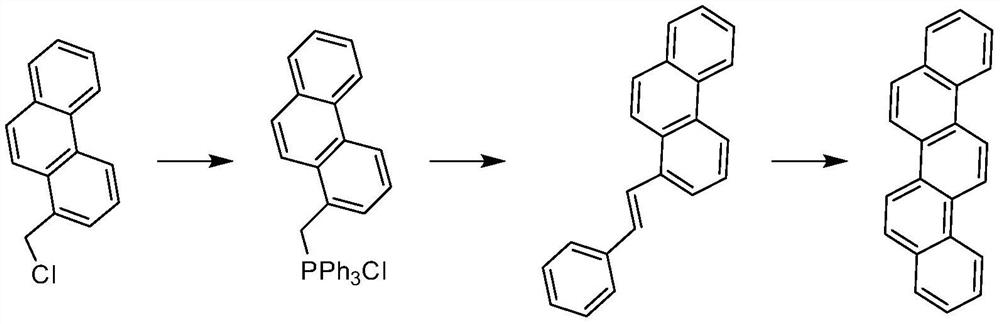
Industrial synthesis method of picene
A technology of benzaldehyde and alkyl, applied in chemical instruments and methods, compounds containing elements of Group 3/13 of the periodic table, preparation of carbon-based compounds, etc., to achieve the effects of high safety, reduced pollution and less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0042] The present invention is described below in conjunction with specific examples, and used raw material, solvent and catalyst are conventional commercially available products, and following examples are used to illustrate the present invention, but are not intended to limit the scope of the present invention.
[0043] A kind of industrial synthesis method of peregrine, is characterized in that, comprises the following steps:
[0044] Step 1: The substrate 2-bromophenanthrene is placed in a low temperature state, and the alkyl derivative of lithium and tributyl borate are subjected to a substitution reaction to obtain the intermediate 2-phenanthrene boronic acid, the 2-bromophenanthrene, the lithium The molar ratio of the alkyl derivative and the tributyl borate is 1:1-2:1-2;
[0045]Step 2: use the intermediate 2-phenanthrene boronic acid and o-bromobenzaldehyde obtained in step 1 to carry out Suzuki coupling reaction to obtain 2-(phenanthrene-2-yl)benzaldehyde, the 2-phe...
Embodiment 1
[0068] The invention discloses a method for the industrial synthesis of peregrine, specifically, the method comprises the following steps:
[0069] Add 2.400kg of the substrate 2-bromophenanthrene (molecular weight 257.13, 9.334mol), THF 200L into a 500L reaction kettle, use liquid nitrogen to lower the temperature of the system to -78℃~-100℃, and control the temperature from -78℃~- 100°C, then add 5.14L (2mol / L, 10.28mol) of n-butyllithium and 2.58kg of tributyl borate (molecular weight: 230.152, 11.211mol) dropwise in sequence to carry out the substitution reaction, stop the reaction when the reaction is complete, and the reaction liquid will heat up naturally To room temperature 20°C-25°C, distill THF off, pour the reaction solution into hydrochloric acid water, solid precipitates, filter to obtain intermediate 2-phenanthreneboronic acid crude product;
[0070] Obtain intermediate 2-phenanthrene boronic acid crude product with step 1 and contain 2-phenanthrene boronic acid ...
Embodiment 2
[0075] The invention discloses a method for the industrial synthesis of peregrine, specifically, the method comprises the following steps:
[0076] Add 2.400kg of the substrate 2-bromophenanthrene (molecular weight 257.13, 9.334mol), THF 200L into a 500L reaction kettle, use liquid nitrogen to lower the temperature of the system to -78℃~-100℃, and control the temperature from -78℃~- 100°C, then add 9.664L (2mol / L, 18.668mol) of n-butyllithium and 4.296kg (molecular weight: 230.152, 18.668mol) of tributyl borate dropwise in order to carry out the substitution reaction, stop the reaction when the reaction is complete, and the reaction liquid will heat up naturally To room temperature 20 ℃ ~ 25 ℃, THF was evaporated, the reaction solution was poured into hydrochloric acid water, the solid precipitated, filtered to obtain the intermediate 2-phenanthrene boric acid crude product;
[0077] Obtain intermediate 2-phenanthrene boronic acid crude product with step 1 and contain 2-phenanth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com