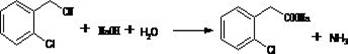Method for synthesizing benzofuran-2 (3H)-ketone
A technology of benzofuran and compounds, which is applied in the field of synthesis of benzofuran-2(3H)-one, can solve the problems of high cost and complicated process, and achieve cost saving, shortening of synthesis steps and improvement of market competitiveness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
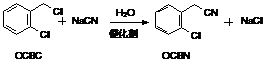
[0050] Embodiment 1, the preparation of o-chlorophenylacetonitrile (OCBN)
[0051] Take a clean reaction bottle, add 5g of catalyst (benzyltriethylammonium chloride) and 270g (1.69mol) of o-chlorochloroben (OCBC) at room temperature, and stir to raise the temperature to 80°C. Add 85g (1.741mol) of 30% sodium cyanide aqueous solution dropwise, the dropping temperature is 80°C, and the drop is completed in about 6-7 hours. After the reaction is qualified, static layering. The water layer goes to the cyanide-containing wastewater treatment device, the oil layer is washed and stratified, and the water layer goes to the cyanide-containing wastewater treatment device. The oil layer is dedistilled, the vacuum degree is controlled not lower than -0.098MPa, and the temperature is not higher than 135°C to obtain the intermediate o-chlorophenylacetonitrile. Yield 95%.
Embodiment 2
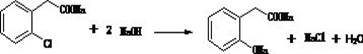
[0052] Embodiment two, the preparation of o-chlorophenyl acetate sodium (OCPANa)
[0053] Add 270g (2.15mol) of sodium hydroxide solution to a clean round-bottomed flask, stir and raise the temperature to 110°C, keep the reflux state, add the intermediate OCBN (1.845mol) dropwise, and the dropwise addition ends in about 4 to 5 hours , after the sampling analysis is qualified, and after the reaction is qualified, the material is dried to obtain solid sodium o-chlorophenylacetate (OCPANa). The ammonia gas produced is absorbed by spraying with aqueous solution containing sulfuric acid. Yield 100%.
Embodiment 3
[0054] Embodiment three, the preparation of o-hydroxyphenylacetic acid (OHPAA)
[0055] At room temperature, add 180g (0.943mol) of the intermediate (OCPANa) dried product, 3g of catalyst copper acetate, and 320g (2.59mol) of liquid caustic soda into the autoclave to dissolve, stir and raise the temperature to 220°C, keep it warm for 2 hours, solution reaction. The pressure is controlled at 1.5-2.1MPa. After the reaction was completed, the temperature was lowered to 110-120°C. Empty, add 80mL of water, filter, and recover the catalyst. The centrifuged mother liquor is transferred to another clean bottle, and 160 g of hydrochloric acid is added for neutralization. The neutralization solution was lowered to room temperature and filtered. The filter cake is washed with water and filtered, and the filter cake is the intermediate o-hydroxyphenylacetic acid (OHPAA). Yield 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com