Preparation method of nettle cyclopeptide in cockscomb seeds and application of nettle cyclopeptide in antitumor drugs
A technology of Urticaceae and cyclic peptides, applied in the field of pharmaceutical technology, anti-tumor compounds, and natural medicinal chemistry, can solve problems such as the mechanism of anti-tumor action of Moroidin, and achieve good anti-tumor activity, wide clinical application prospects, and dosage forms and the effect of diversification of medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
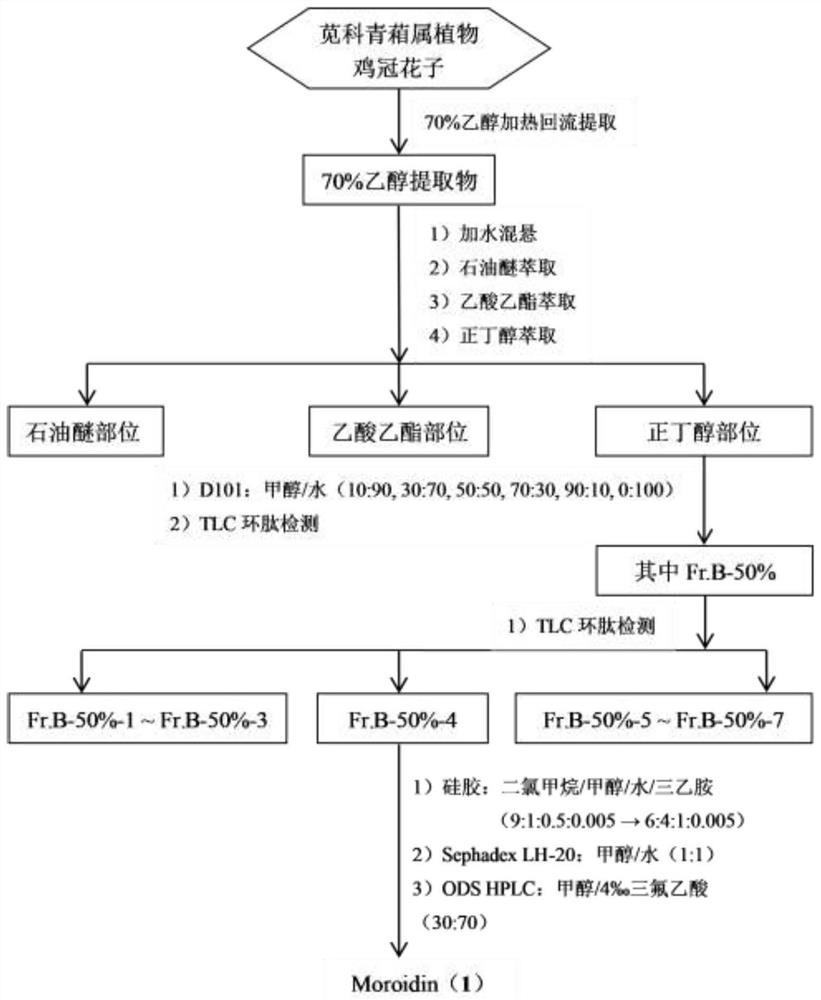
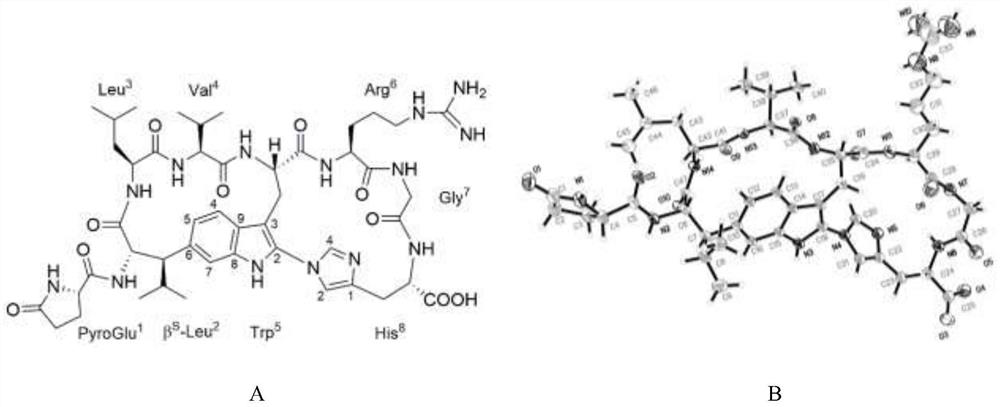
[0029] The preparation method of embodiment 1 Urticaceae type cyclic peptide Moroidin (1) (flow chart sees figure 1 shown).
[0030] Take dried cockscomb flower seeds (30kg), heat and reflux extraction with 10 times the volume of 70% ethanol after crushing, extract three times (30L×3), each time for 3 hours, combine the extracts, and concentrate under reduced pressure to obtain the total Extract (1.05kg). Suspend the total extract in water, extract continuously with petroleum ether, ethyl acetate, n-butanol respectively, extract three times with equal volume, reclaim the solvent to obtain petroleum ether part (33g), ethyl acetate part (60g), n-butanol Part (Fr.BuOH, referred to as Fr.B, 413g) and the water part after extraction.
[0031] The fraction of n-butanol (Fr.B, 413g) was chromatographed by D101 macroporous adsorption resin column, with methanol / water system (10:90, 30:70, 50:50, 70:30, 90:10, 0:100 , v / v) gradient elution, wherein 50% of the methanol / water system (...
Embodiment 2
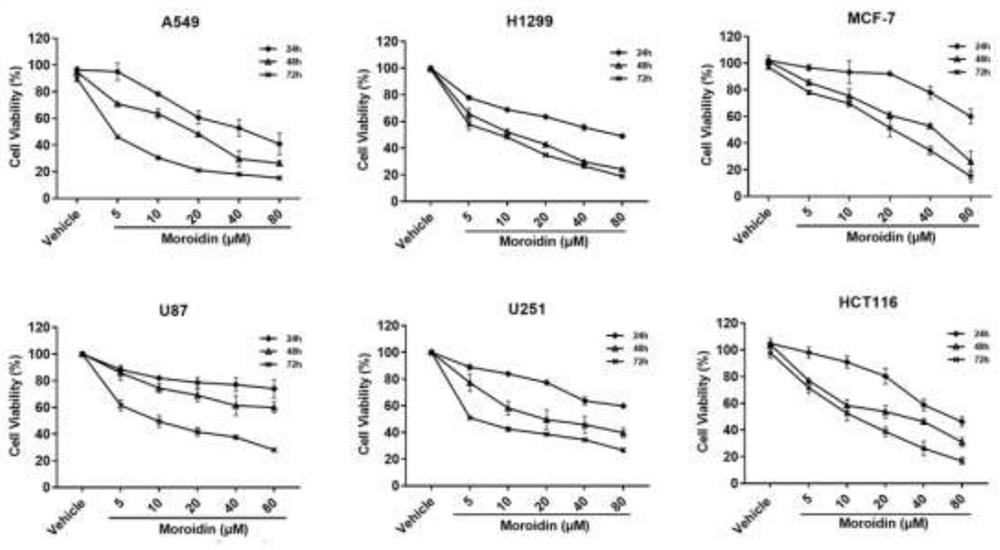
[0040] Cytotoxic effect of the Urticaceae type cyclic peptide Moroidin prepared in Example 2 on tumor cells
[0041] Experimental method: using thiazolyl blue (3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide, MTT) experiment, plant cyclic peptide Moroidin (concentration gradient is 5 μM, 10 μM , 20 μΜ, 40 μΜ, 80 μΜ) to treat lung cancer cells A549, H1299, breast cancer cells MCF7, glioma cells U87, U251, and colorectal cancer cells HCT116, and analyze the in vitro anti-tumor activity of plant cyclic peptide Moroidin. The specific steps are: (1) Cell sorting: Tumor cells in good logarithmic growth phase were inoculated into 96-well plates at an inoculation concentration of 5000 cells / well, and cultured in a conventional cell incubator for 12 hours. (2) Administration: The culture medium was discarded, and the experimental groups were given different concentrations of plant cyclic peptide Moroidin (5 μM, 10 μM, 20 μM, 40 μM, 80 μM), and 5 replicate wells were set for ...
Embodiment 3
[0043] Example 3 Effect of Urticaceae Cyclic Peptide Moroidin on Long-term Proliferation of Tumor Cells
[0044] Experimental method: The plant cyclic peptide Moroidin of the present invention acts on lung cancer cell A549 and breast cancer cell MCF7 at different concentrations, and detects the long-term growth of tumor cells through a clone formation experiment.
[0045] The tumor cells in the logarithmic growth phase were evenly seeded in 6-well plates, with 500 cells per plate. After the cells adhered to the wall, they were treated with different concentrations of Moroidin for 7-10 days, and the cell colonies were stained with 0.1% crystal violet solution for 2 minutes. The background color was eluted with PBS, and then photographed. Use the Image Tool software to calculate the number of clones, and calculate the cloning rate with the following formula: cloning rate=(forming the number of clones / the number of planted cells)×100%
[0046] Experimental results: as attached ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com