Method for preparing fluoroalkane compound from fluorinated epoxide
A technology of oxides and fluorinated rings, applied in the preparation of hydroxyl compounds, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of high risk, increased by-products, and difficult industrialization, and achieves less by-products, Simple operation, safe and controllable effect of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
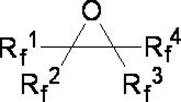
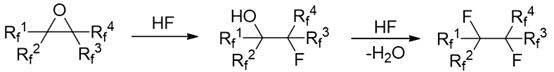
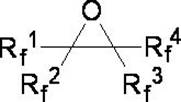
[0025] Example 1: Preparation of perfluorohexane from perfluoro-2-methyl-2,3-epoxypentane.
[0026] In a dry and clean 1L autoclave, add 100g of diethylene glycol dimethyl ether, 241.5g (1.5mol) of triethylamine hydrogen fluoride salt, and 316g of perfluoro-2-methyl-2,3-epoxypentane ( 1mol); close the reaction kettle, turn on the reaction kettle to stir, gradually increase the reaction temperature to 130°C, stir and react for 24 hours, after the reaction is completed, cool to room temperature, take out the reaction solution, and wash the reaction solution twice with a mass concentration of 10% sodium carbonate solution. Separation, the lower organic phase was obtained and washed three times with pure water, and the crude product was obtained by liquid separation. After the crude product was purified by rectification, 307.92 g of the pure product perfluorohexane was obtained, and the reaction yield was 91.1%.
Embodiment 2
[0027] Example 2: Perfluoro-4-methyl-3-isopropyl-2,3-epoxypentane Preparation of perfluoro-2-methyl-3-isopropylpentane.
[0028] In a dry and clean 1L autoclave, add 100g of diethylene glycol dimethyl ether, 241.5g (1.5mol) of triethylamine hydrogen fluoride, perfluoro-4-methyl-3-isopropyl-2,3- 466g (1mol) of pentylene oxide, close the reaction kettle, start the reaction kettle to stir, gradually increase the reaction temperature to 180°C, stir and react for 24h, after the reaction is completed, cool to room temperature, take out the reaction solution, and use a mass concentration of 10% sodium carbonate solution Wash the reaction solution twice, separate the liquids, and wash the lower organic phase with pure water three times, separate the liquids to obtain the crude product, and obtain the pure product perfluoro-2-methyl-3-isopropylpentane after the crude product is purified by rectification Alkane 437.74g, reaction yield 89.7%.
Embodiment 3
[0029] Example 3: The operation steps are the same as in Example 1, except that the hydrogen fluoride source is changed from triethylamine hydrogen fluoride to dimethylamine hydrogen fluoride.
[0030] In a dry and clean 1L autoclave, add 100g of diethylene glycol dimethyl ether, 127.5g (1.5mol) of dimethylamine hydrogen fluoride, and 316g of perfluoro-2-methyl-2,3-epoxypentane ( 1mol). Close the reaction kettle, turn on the reaction kettle to stir, gradually increase the reaction temperature to 130°C, stir and react for 24 hours, after the reaction is completed, cool to room temperature, take out the reaction solution, wash the reaction solution twice with a mass concentration of 10% sodium carbonate solution, and separate the liquid. The obtained lower organic phase was washed three times with pure water, and the crude product was obtained by liquid separation. After the crude product was purified by rectification, 298.92 g of the pure product perfluorohexane was obtained, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com