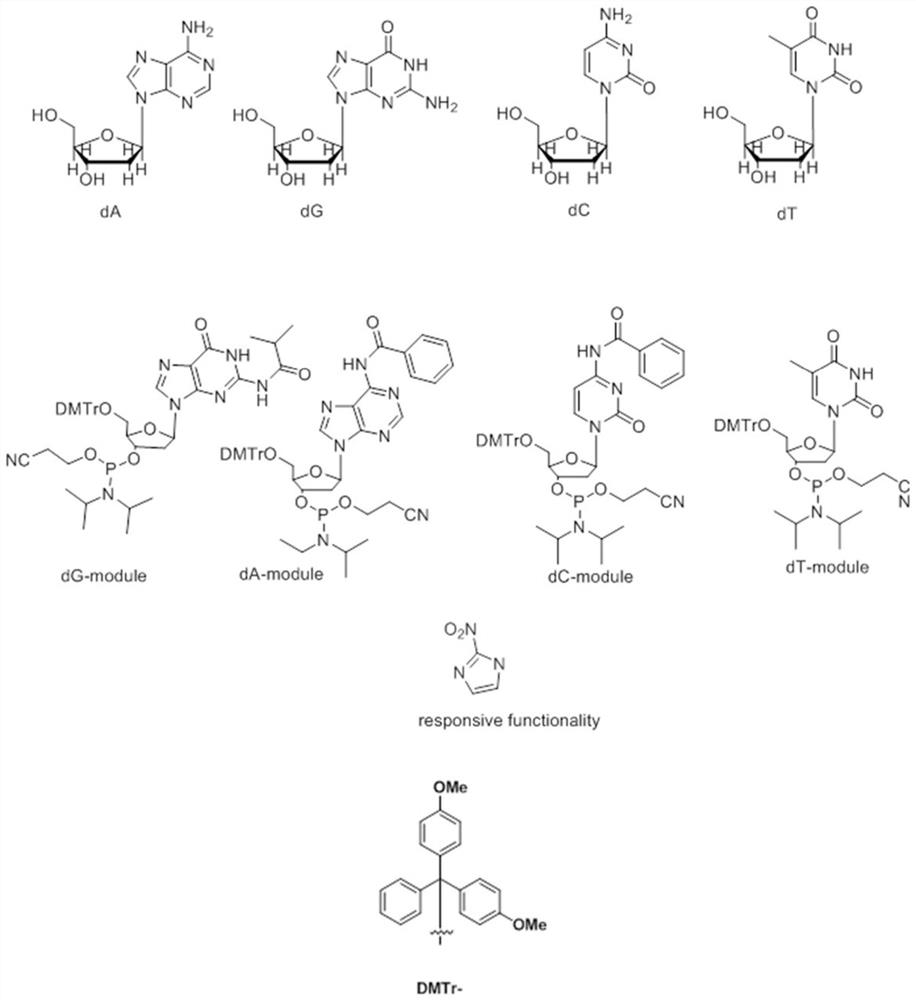
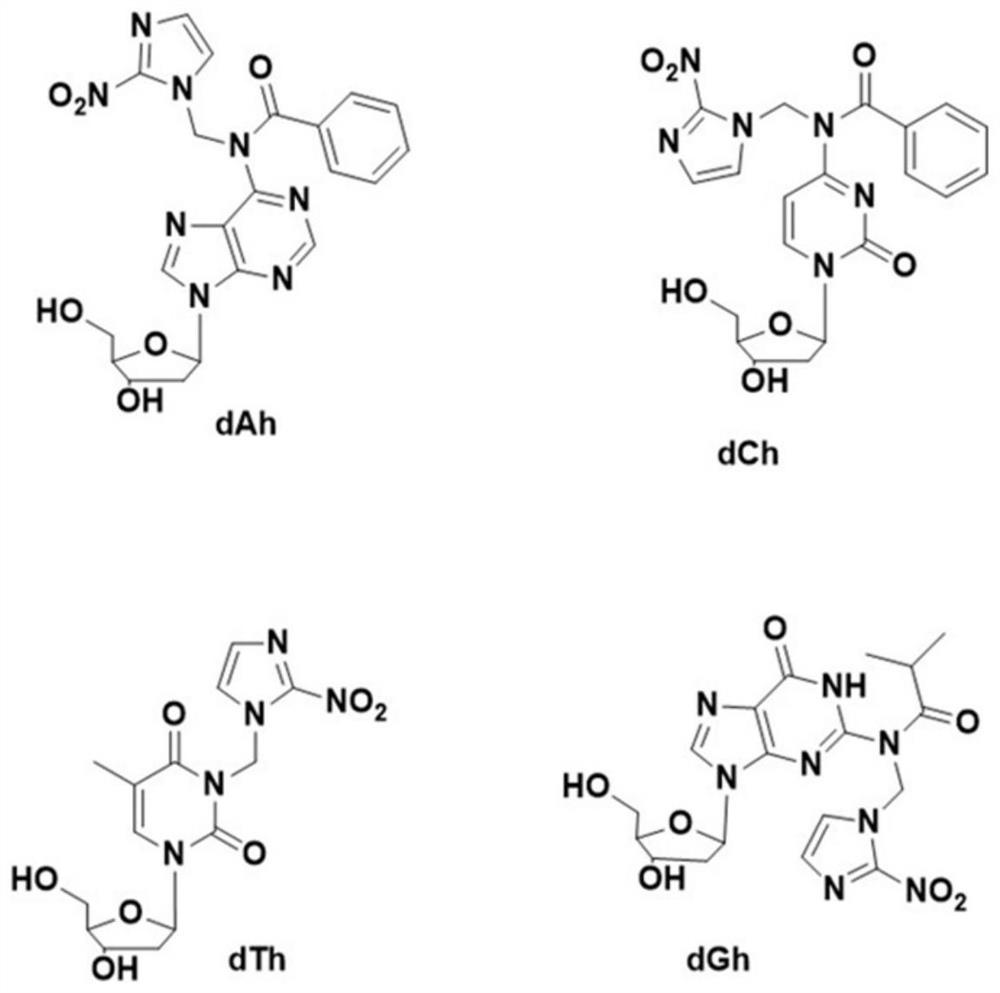
Artificial deoxyribose responding to low-oxygen microenvironment and application of artificial deoxyribose
A nucleotide and cycloalkyl technology, applied in artificial deoxyribose and its application in cancer diagnosis and treatment, can solve the problems of unsatisfactory targeting and biological stability in low-oxygen microenvironment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0082] In the preparation method of the present invention, each reaction is usually carried out in an inert solvent at a reaction temperature of -78°C to 150°C (preferably 20 to 120°C). The reaction time of each step is usually 0.5-48 hours, preferably 2-12 hours.
[0083] Pharmaceutically acceptable salts, solvates, stereoisomers, tautomers
[0084] As used herein, the term "pharmaceutically acceptable salt" refers to the salts formed between the compounds of the present invention and pharmaceutically acceptable inorganic and organic acids, wherein preferred inorganic acids include (but are not limited to): hydrochloric acid, hydrogen Bromic acid, phosphoric acid, nitric acid, sulfuric acid; preferred organic acids include (but are not limited to): formic acid, acetic acid, propionic acid, succinic acid, naphthalene disulfonic acid (1,5), subacidic acid, oxalic acid, tartaric acid, lactic acid , salicylic acid, benzoic acid, valeric acid, diethylacetic acid, malonic acid, succ...
Embodiment 1
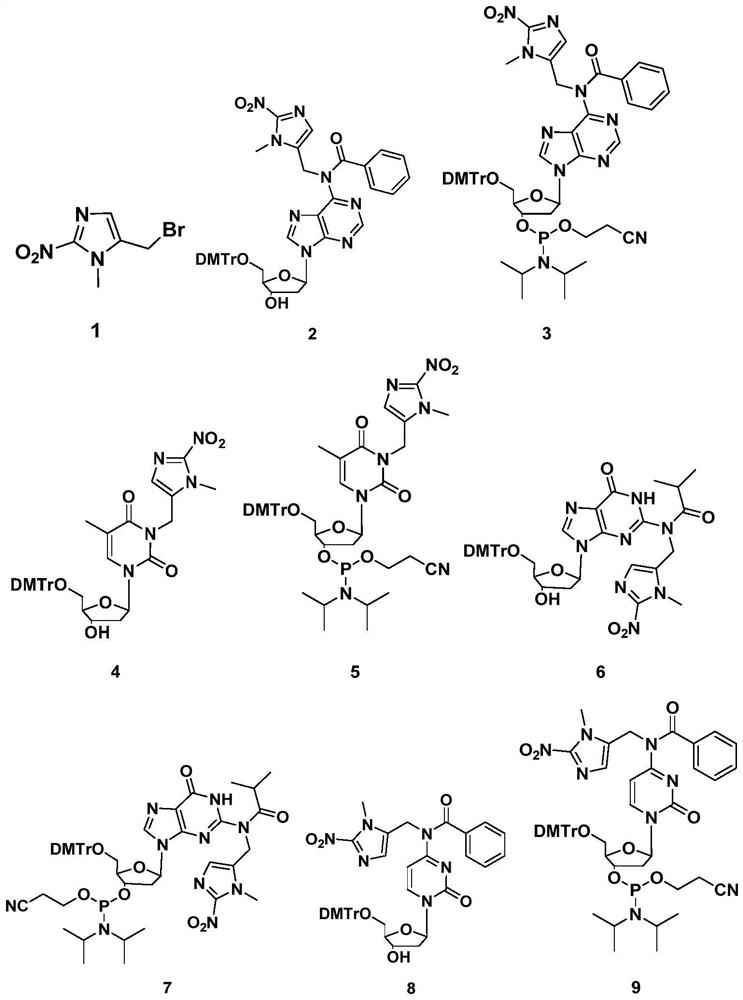
[0107] Example 1 Preparation of a stress-responsive deoxyribose probe based on 5-(bromomethyl)-1-methyl-2-nitro-1H-imidazole
[0108] 1.1 Preparation of compound 1 (5-(bromomethyl)-1-methyl-2-nitro-1H-imidazole)
[0109] The specific operation process is as follows:
[0110]
[0111] Under the protection of nitrogen, slowly add PPhBr 3 (19 mmol) and maintained at 0° C. for 5 hours. After the reaction was monitored by TLC, the solvent was concentrated under reduced pressure to remove the solvent, and the target compound was separated by column chromatography with high yield (yield 81%).
[0112] Compound 1: (5-(Bromomethyl)-1-methyl-2-nitro-1H-imidazole):
[0113] 1 H NMR (500MHz, CDCl 3 ), δ (ppm) = 7.21 (s, 1H), 4.50 (s, 2H), 4.07 (s, 3H); 13 C NMR (126MHz, CDCl 3 ), δ (ppm) = 152.20, 133.08, 128.35, 34.07, 18.95. MS (ESI) m / z for C 5 h 6 BrN 3 o 2 :218.9643 (calcd.), 218.9651 (expt.).
[0114] 1.2 Preparation of Compound 2
[0115]
[0116] Under nitrogen p...
Embodiment 2
[0155] Example 2 Preparation of a stress-responsive deoxyribose probe based on 2-(bromomethyl)-1-methyl-5-nitro-1H-imidazole
[0156] 2.1 Preparation of compound 10 (2-(bromomethyl)-1-methyl-5-nitro-1H-imidazole)
[0157]
[0158] Under the protection of nitrogen, slowly add PPhBr 3 (19 mmol), and maintained at 0 ° C for 30 min, then slowly rose to room temperature to continue the reaction for 5 h. After the reaction was monitored by TLC, the solvent was concentrated under reduced pressure to remove the solvent, and the target compound was obtained by column chromatography with high yield (yield 85%).
[0159] Compound 10: 2-(Bromomethyl)-1-methyl-5-nitro-1H-imidazole:
[0160] 1 H NMR (500MHz, CDCl 3 ), δ (ppm) = 7.81 (s, 1H), 4.50 (s, 2H), 3.82 (s, 3H); 13 C NMR (126MHz, CDCl 3 ), δ (ppm) = 162.08, 139.44, 136.35, 34.06, 28.85; MS (ESI) m / z for C 5 h 6 BrN 3 o 2 :218.9643 (calcd.), 218.9655 (expt.).
[0161] 2.2 Preparation of compound 11
[0162]
[0163] U...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com