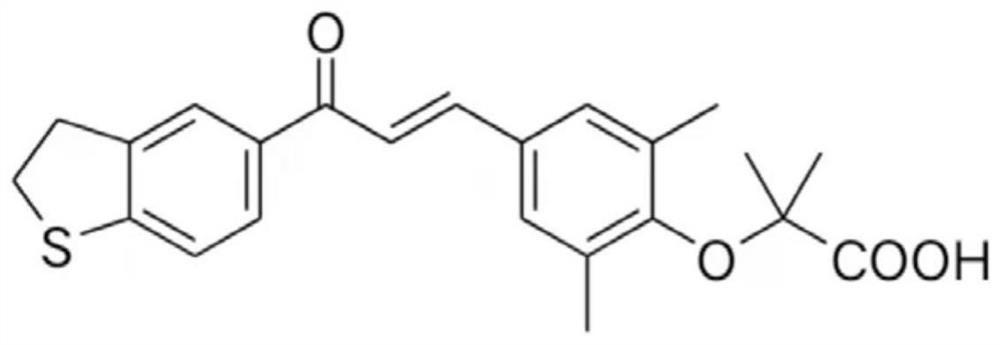
Application of LZ01 in treatment of Duchenne muscular dystrophy
A Duchenne muscular dystrophy and dystrophy technology, applied in the field of medicine, can solve problems such as no reports of LZ01 treating Duchenne muscular dystrophy, and achieve the effects of improving skeletal muscle pseudohypertrophy, muscle weight reduction, and reduced infiltration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Pharmacodynamic evaluation of LZ01 in the treatment of muscle weakness and muscle atrophy in 3-4 weeks old mdx mice. Named as DMD model group, simvastatin treatment group and LZ01 treatment group, the doses of simvastatin treatment group and LZ01 treatment group were 20 mg / kg / day and 30 mg / kg / day, respectively. The specific groups and administration methods are shown in Table 1:
[0034] Table 1 Specific grouping of experimental mice, dosage and mode of administration
[0035]
[0036] Drugs were administered at 9-10 am every day, and all animal states were observed and recorded.
[0037] Muscle grasping force determination; for 3 weeks and 6 weeks after administration, the forelimb grasping force of mice was measured. The average value of each test was taken 3 times, and the results are shown in Table 2.
[0038] Table 2 Statistical results of muscle grip in mdx mice
[0039] grouping DMD model group LZ01 treatment group Simvastatin treatment gro...
Embodiment 2
[0043] Fasting after the last administration, the mdx mice in Example 1 were euthanized 24 hours later, blood was collected from the inguinal vein, centrifuged at 3500 rpm for 10 minutes, and the upper serum was taken to measure serum creatine kinase (CK) and lactate dehydrogenase (LDH) content. The gastrocnemius muscle (GAS), tibialis anterior muscle (TA), and diaphragm muscle (DIA) were then dissected and quickly frozen in liquid nitrogen, and then transferred to a -80°C refrigerator for use the next day. Serum CK, LDH content and skeletal muscle coefficient are shown in Table 3.
[0044] Table 3 mdx mouse skeletal muscle coefficient
[0045]
[0046]
[0047] * P<0.05 compared with DMD model group.
[0048] The results showed that after administration of LZ01, the content of serum creatine kinase tended to increase, but it had little effect on the content of LDH, and further research was needed. In addition, LZ01 had a tendency to reduce muscle weight in mdx mice,...
Embodiment 3
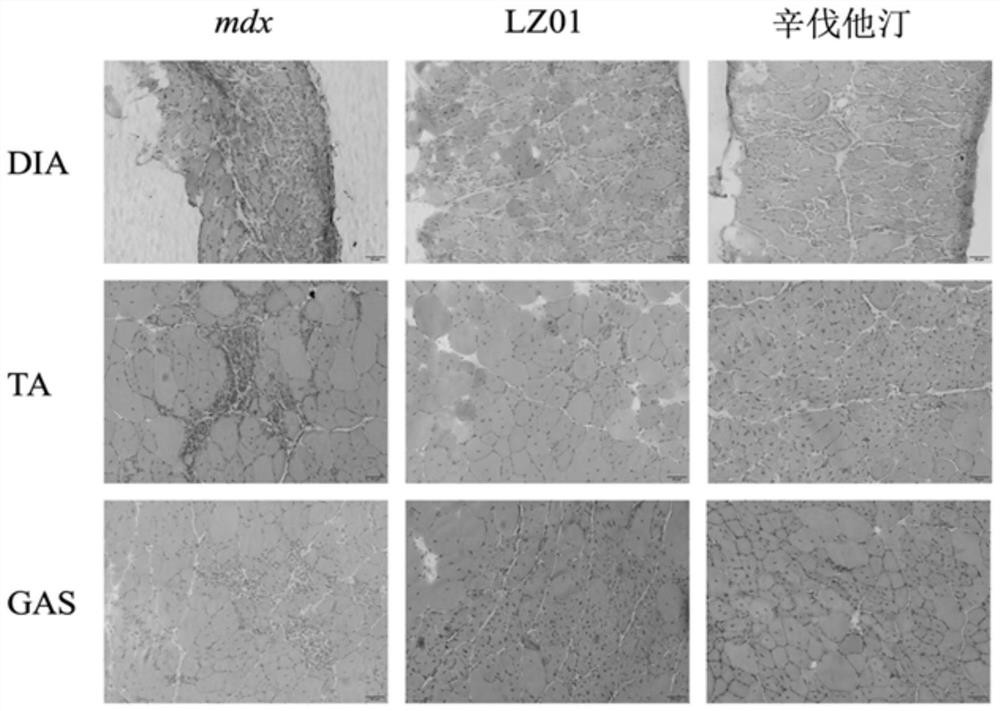
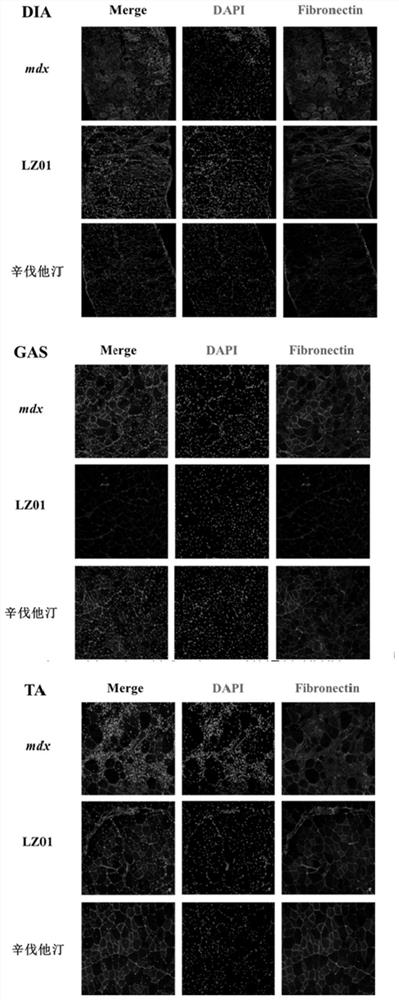
[0050] Hematoxylin-eosin (HE) staining was used to observe the pathological and morphological changes of skeletal muscle of each group of experimental mice in Example 3: fresh gastrocnemius muscle, tibialis anterior muscle and diaphragm muscle were quickly OCT embedded, and then sliced in a cryostat. Each group of sections was stained with HE dye, and after mounting, the sections were observed and photographed under a microscope of model BX53 manufactured by Olympus in Japan. The result is as figure 2 shown.
[0051]The results showed that a large number of inflammatory cells were infiltrated in the tibialis anterior muscle and diaphragm muscle of the mice in the DMD model group, and the muscle fibers became rounded with different sizes, the nucleus was centralized, and the muscle fibers were obviously lost. LZ01 administration significantly increased the number of muscle fibers in the gastrocnemius, tibialis anterior and diaphragm muscles of mdx mice, and alleviated the n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com