RTG fusion protein capable of enhancing immunogenicity and immune protection effect
A technology for immune protection and immune enhancement, applied in the field of genetic engineering, can solve the problems of weak cellular immune response and achieve the effect of improving ability and good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
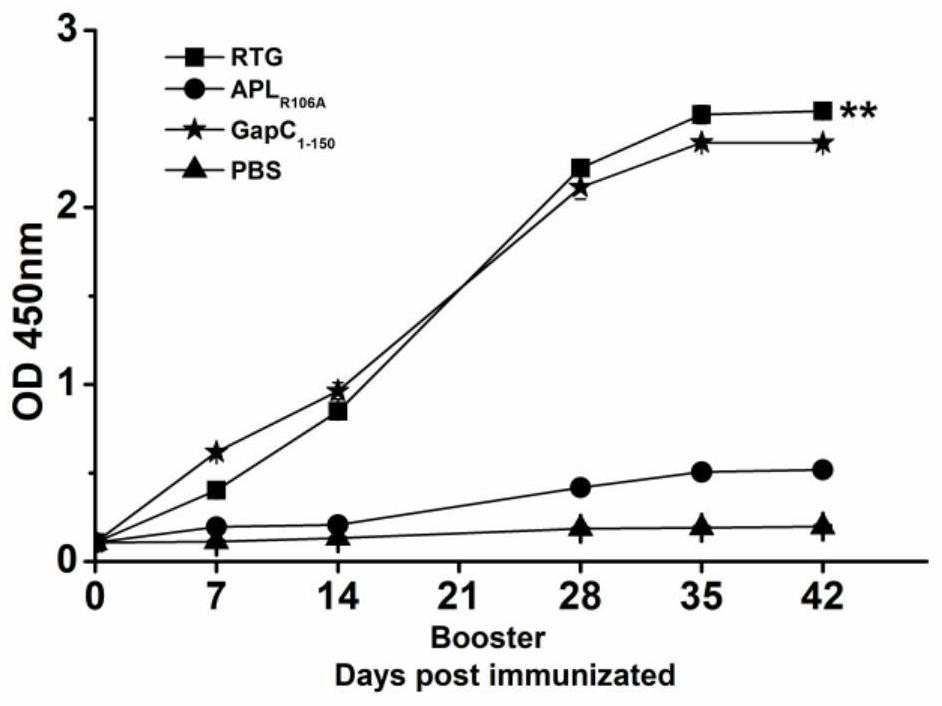
[0024] This example illustrates the immunogenicity of fusion protein RTG.
[0025] 1. Construction of recombinant fusion protein
[0026] According to the amino-terminal sequence of Streptococcus agalactiae GapC published by Genebank (ID: AAM73770.1), the GSGSGSGS protein flexible Linker was used to convert APL R106A The amino acid sequence of (RNFGGFKSYRLLAPAKGTTY) is concatenated with amino acids 1-150 of GapC of Streptococcus agalactiae to form a recombinant fusion protein APL R106A -GapC 1-150 , named RTG (such as figure 1 shown). Codon optimization was used for codon optimization, and Primer 5.0 was used to design specific upstream and downstream primers. The BamH I restriction site was introduced at the 5' end of the upstream primer, and the Xho I restriction site was introduced at the 3' end of the downstream primer. The primer sequences are shown in Table 1, and the underlines represent restriction enzyme cleavage sites. The target gene was amplified by PCR and co...
Embodiment 2
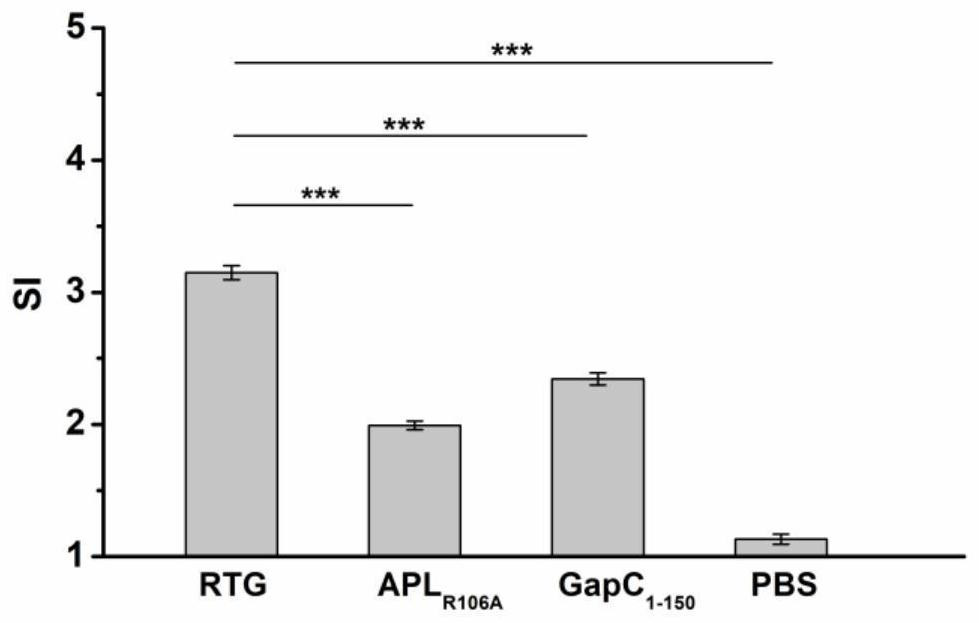
[0044] This example illustrates the immunoprotective effect of fusion protein RTG.
[0045] 1. Immunization of experimental animals
[0046] SPF grade 4-6 weeks old female BALB / c mice were randomly divided into RTG experimental group, APL R106A Experimental group, GapC 1-150 The experimental group and the PBS control group were given free access to food and water. After a week of feeding, the purified protein was emulsified with Freund's complete adjuvant in a volume ratio of 1:1, and injected into the leg muscle by intramuscular injection into each mouse. 50 μg, three weeks after the primary immunization, the same amount of immunogen was emulsified with incomplete Freund's adjuvant and the mice were immunized again. PBS was simultaneously emulsified with the same adjuvant as a control.
[0047] 2. Culture of strains and challenge test of experimental animals
[0048] Take out the glycerol bacteria Streptococcus agalactiae HLJ-6 strain and Staphylococcus aureus Newman stra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com