Synthesis method and application of 1, 3-aza-silane compound
A technology of azasilane and synthesis method, which is applied in the field of chemical synthesis of silicon nitrogen compounds, can solve the problems of harmful transition metal catalysts, difficult substrate synthesis, and limited application range, and achieves non-strict reaction conditions, low risk, and applicable strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
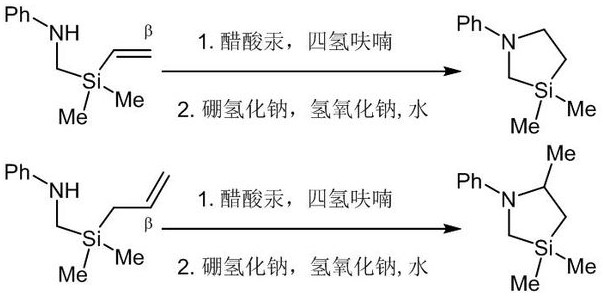
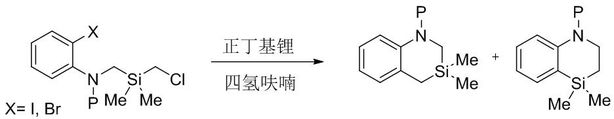
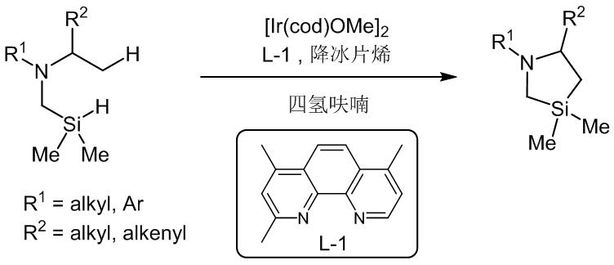
Image
Examples
Embodiment 1
[0092] A method for synthesizing a class of 1,3-azasilane compounds, comprising the following steps:
[0093] A. The compound represented by formula (I) is sequentially subjected to nucleophilic substitution reaction with the compound represented by formula (II) and / or the compound represented by formula (III) to obtain the compound represented by formula (IV);
[0094] b. Under basic conditions, the obtained compound represented by the formula (IV) is subjected to a nucleophilic substitution reaction with the compound represented by the formula (V); then the Boc protecting group is removed to obtain the compound represented by the formula (VI), namely 1 ,3-azasilane compound;
[0095] The reactions involved are as follows:
[0096]
[0097] where R 1 is methyl or chloro, R 2 is methyl or chloro, R 3 is alkyl, alkenyl, alkynyl or aryl, R 4 is benzyl or aryl, R 5 is alkyl, alkenyl, alkynyl or aryl, R 6 is sulfonyl, acyl, alkyl or aryl.
[0098] When the reaction sub...
Embodiment 2
[0121] A kind of 1,3-azasilane compound synthesized by the above method is used for preparing silinoindoline.
[0122] The 1,3-azasilane compound is used to prepare the silinoindoline, which specifically includes: under basic conditions, the compound represented by the formula (VI) and the compound represented by the formula (VII) are subjected to a [3+2] ring chemical reaction to obtain the compound represented by formula (VIII), namely silinoindoline;
[0123] The reactions involved are as follows:
[0124]
[0125] where R 7 is an alkyl group, an alkenyl group, an alkynyl group, an aryl group or a halogen atom.
[0126] In the [3+2] cyclization reaction, the base is one or more combinations of cesium fluoride, potassium fluoride, potassium carbonate, cesium carbonate and potassium hydroxide;
[0127] Adopting two-phase catalyst is one or more combinations of 18-crown-6, 15-crown-5, benzo-18-crown-6 and dibenzo-18-crown-6;
[0128] The reaction solvent is one or a com...
Embodiment 3
[0131] Based on Embodiment 2, this embodiment also provides: a product containing the silinoindoline synthesized by the above method, including the silinoindoline compound or a pharmaceutically acceptable salt thereof as an active ingredient, and a pharmaceutically acceptable product A pharmaceutical composition consisting of a carrier, diluent and excipient.
[0132] More specifically, for example, the product is an analgesic, antitumor, antibacterial, antidepressant, or vasodilator.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com