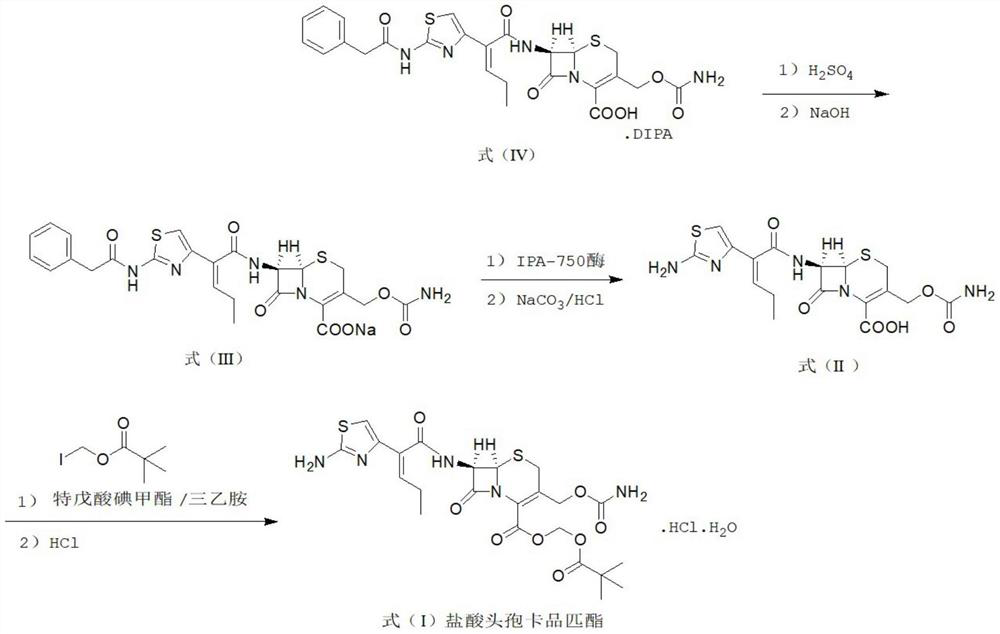
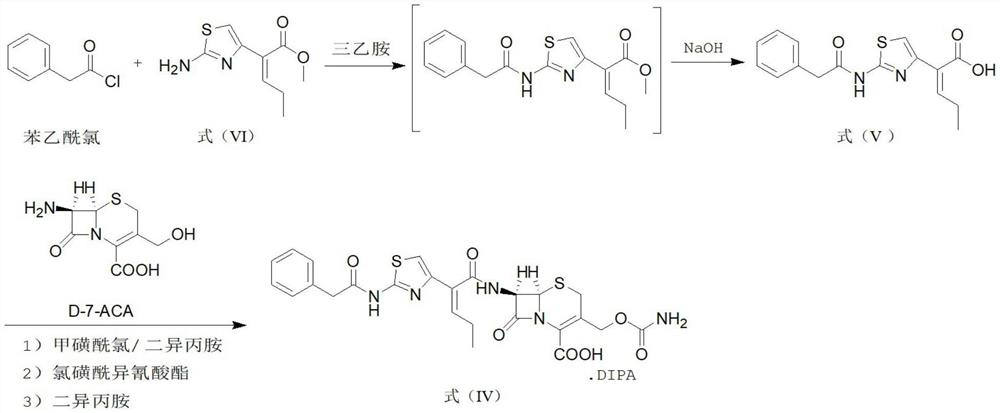
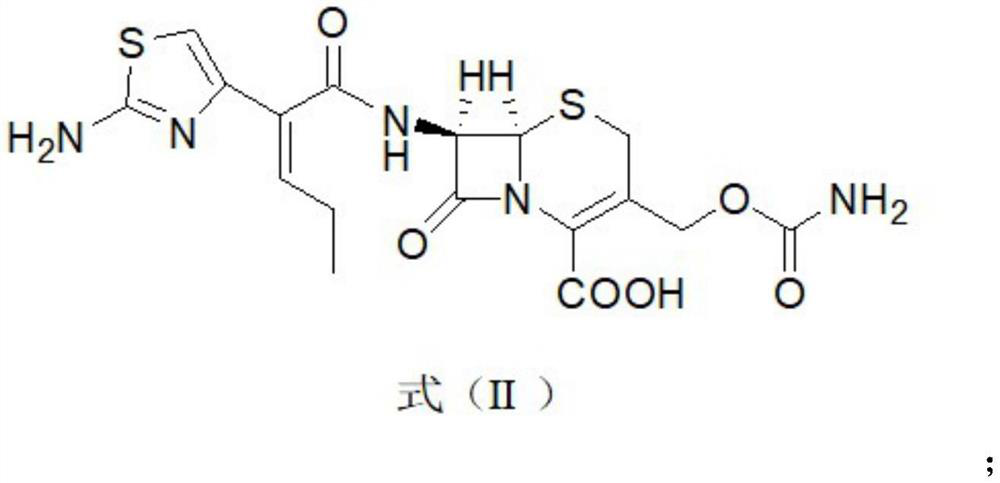
Method for synthesizing cefcapene pivoxil hydrochloride by using enzyme method and synthesized intermediate of cefcapene pivoxil hydrochloride
A technology of cefcapine and enzymatic synthesis, applied in organic chemistry, fermentation, etc., can solve the problems of unstable cephalosporin antibiotic compounds, difficulty in removing phenol impurities, and easy occurrence of side reactions, so as to facilitate industrial production and improve product purity , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] The preparation of compound shown in embodiment 1 formula (V)
[0115] At room temperature, 200 mL of dichloromethane was added to a clean reaction flask, and then 15 g (0.07 mol, 1 eq) of the compound of formula VI was added, and the mixture was stirred to dissolve. The material was gradually cooled, and the temperature was controlled at 5-10° C., 17.3 g (0.112 mol, 1.6 eq) of phenylacetyl chloride was added, and the mixture was stirred uniformly. Then, the temperature was controlled at 5-10° C., and 12.0 g (0.119 mol, 1.7 eq) of triethylamine was added dropwise. After the dropwise addition, the temperature was controlled at 5-10°C, and the acylation reaction was stirred for 3 hours. After the reaction, 1.5% dilute hydrochloric acid solution was added to the feed solution, the pH value was adjusted to be neutral, the temperature was controlled at 10-15° C., stirred and washed for 5 minutes, and stood for 30 minutes for liquid separation. The aqueous layer was discard...
Embodiment 2
[0120] The preparation of compound shown in embodiment 2 formula (IV)
[0121] At room temperature, 150 mL of dichloromethane was added to a clean three-necked flask, and 18.0 g (0.057 mol, 1.0 eq) of the compound represented by formula (V) and 7.8 g (0.068 mol, 1.2 eq) of methanesulfonyl chloride were added, and the mixture was stirred. Cool down to 0°C. The temperature was controlled at 0°C-10°C, 7.5 g (0.074 mol, 1.3 eq) of diisopropylamine was added, and the reaction was stirred for 30 minutes. After the reaction, the reaction solution A was obtained, which was kept at 0°C to 10°C for use. At room temperature, 100 mL of methanol was added to another clean three-necked flask, 13.1 g (0.057 mol, 1.0 eq) of D-7-ACA was added, stirred and cooled to 0°C. The temperature was controlled at 0°C-10°C, 6.4 g (0.063 mol, 1.1 eq) of diisopropylamine was added, and the reaction was stirred for 20 minutes. After the reaction was completed, a reaction solution B was obtained, and the ...
Embodiment 3
[0122] Example 3 Preparation of compound represented by formula (II)
[0123] At room temperature, 200 mL of dichloromethane and 150 mL of water were added to a dry and clean three-necked flask, and 30.0 g (0.045 mol, 1.0 eq) of the compound of formula IV was added, stirred and cooled to 10°C. Control the temperature below 15°C, add 10% sulfuric acid solution, adjust the pH value to 4.0-4.5, and stir to dissolve. Let stand for 30 minutes. The aqueous layer was discarded during liquid separation, and the organic layer feed liquid was collected for use. Add 200 mL of water to the organic layer, control the temperature to 10-15° C., add 10% aqueous sodium hydroxide solution, adjust the pH to 7.0-7.5, and stir for 10 minutes. Let stand for 30 minutes. During the separation, the organic layer was discarded, and the aqueous solution of the compound of formula (III) was collected for use. The temperature was controlled at 25°C-30°C, 15 g of IPA-750 enzyme was added to the above-m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com