CDC7 inhibitor related substance detection method
A technology related to substances and detection methods, applied in the field of drug analysis, can solve problems such as the lack of detection methods, and achieve the effects of stable stability, simple operation and strong adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
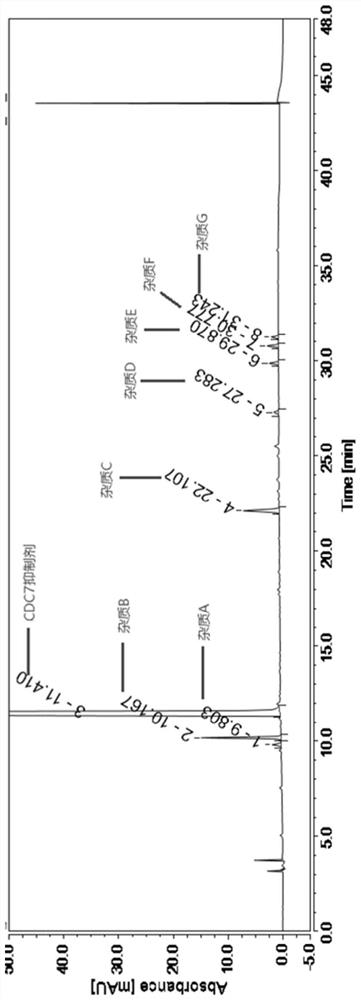
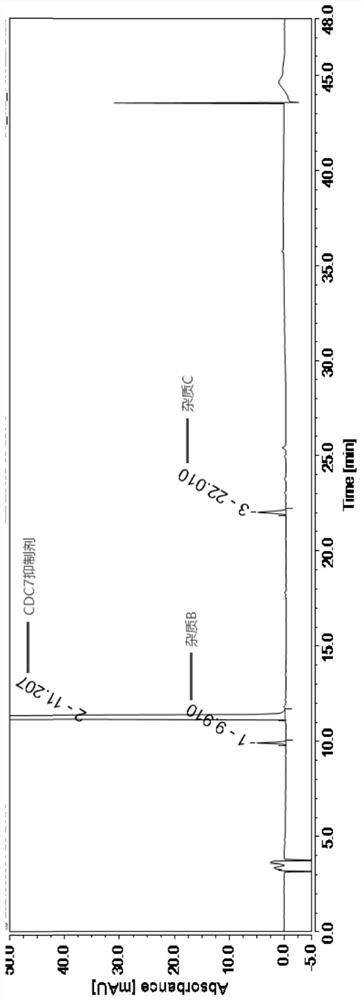
[0036] Embodiment 1: specificity test.
[0037] The specificity test needs to verify that the blank solution has no interference at the retention time of the main peak in the test product and the reference solution and the resolution between impurities and the main component. The following is the preparation method of each impurity and main component:
[0038] Impurity A positioning solution: Accurately weigh 17.5 mg of the impurity A reference substance, add methanol: water (1:1) to a 50ml measuring bottle, ultrasonically dissolve and dilute to the volume, shake well, and use it as the impurity A stock solution; The impurity A stock solution was quantitatively diluted to make 17.5 μg / ml, which was used as the impurity A positioning solution.
[0039] Impurity B positioning solution: Accurately weigh 17.5mg of the impurity B reference substance, put it into a 50ml measuring bottle, add methanol: water (1:1) ultrasonically to dissolve and dilute to the volume, shake well, and ...
Embodiment 2
[0057] Embodiment 2: Sensitivity test.
[0058] Take the impurity B stock solution, impurity C stock solution, and reference substance solution prepared in Example 1 and dilute with a solvent to make a solution with a suitable concentration, dilute step by step to a suitable multiple, and use the solution when the signal-to-noise ratio ≥ 10:1 as the limit of quantitation Solution; the solution when the signal-to-noise ratio ≥ 3:1 is used as the detection limit solution.
[0059] Precisely measure 10 μl each of the limit of quantification solution and the limit of detection solution, and inject them into the liquid chromatograph.
[0060] Table 2 Quantitative limit verification results
[0061] name Concentrationμg / ml S / N Sensitivity (%) Impurity B 0.105540 22.0 0.03 CDC7 inhibitor 0.104796 11.3 0.03 Impurity C 0.095247 18.1 0.03
[0062] Table 3 Detection limit verification results
[0063] name Concentrationμg / ml S / ...
Embodiment 3
[0065] Embodiment 3: repeatability test
[0066] The test solution: take an appropriate amount of CDC7 inhibitor, weigh it accurately, add solvent to dissolve and dilute to make a solution containing 0.35mg per 1ml. Prepare 6 copies in parallel.
[0067] Accurately measure each 10 μl of the test solution respectively and inject it into a liquid chromatograph. The chromatographic conditions are the same as in Example 1. The results are shown in Table 4.
[0068] Table 4 Repeatability inspection results
[0069] Impurity No. 1 2 3 4 5 6 RSD(%) Impurity B 0.12 0.11 0.11 0.11 0.11 0.11 3.66 Impurity C 0.16 0.16 0.16 0.16 0.16 0.16 0.00 total miscellaneous 0.07 0.07 0.07 0.07 0.07 0.07 0.00
[0070] It can be seen from Table 4 that the RSD of single impurity content meets the requirements (the RSD of each impurity with a content of less than 0.5% is not greater than 10.0%; the RSD of each impurity with a content of 0.5% to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Column length | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com