Preparation method of gadobutrol and intermediate thereof
An ethanol, reaction time technology, applied in organic chemistry, bulk chemical production, etc., can solve problems such as non-compliance with regulatory requirements, elimination, risks, etc., to avoid drug safety and/or environmental pollution, less by-products, The effect of mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078]1. Prepare DO3A-T-BU-ESTER
[0079]
[0080] These include the following steps:
[0081] (1), the 10g (about 58mmol) cyclen (Name: 1,4,7,10-tetraazacyclododecane) and 15.71g (about 191.6mmol) sodium anhydrous acetate were added to 90mL DMF (N, N-dimethylformamide), and then added 40.76g (about 209mmol) tert-butyl bromoacetate and 30mL DMF mixed solution, reacted at 20 ~ 30 °C for 6h, to give the reaction solution;
[0082] (2), add 330mL of purified water to the resulting reaction solution, and then adjust the pH value to 8.8~9.0 with an aqueous sodium carbonate solution (mass percentage concentration of 8.0%), pump filtration, and the filter cake is rinsed twice with purified water (20mL×2) to obtain wet products; The wet product (about 30g) was added to 90mL ethanol, heated to 40 ~ 45 ° C, dissolved, and then added 270mL of purified water, stirred at room temperature for 2h, filtered, filter cake with purified water twice (20mL×2), 50 ~ 60 ° C blast dried, to give the pr...
Embodiment 2~4
[0115]The same content as Example 1 is no longer repeated, the difference is that in the step (1) of the DO3A-T-BU-ESTER preparation method, the anhydrous sodium acetate is changed to potassium carbonate, lithium hydroxide, sodium bicarbonate (the dosage is 191.6mmol, i.e., 3.3 equivalents), the reaction liquid is obtained, and then subsequent separation and purification are carried out according to the same method to obtain the product DO3A-T-BU-ESTER.
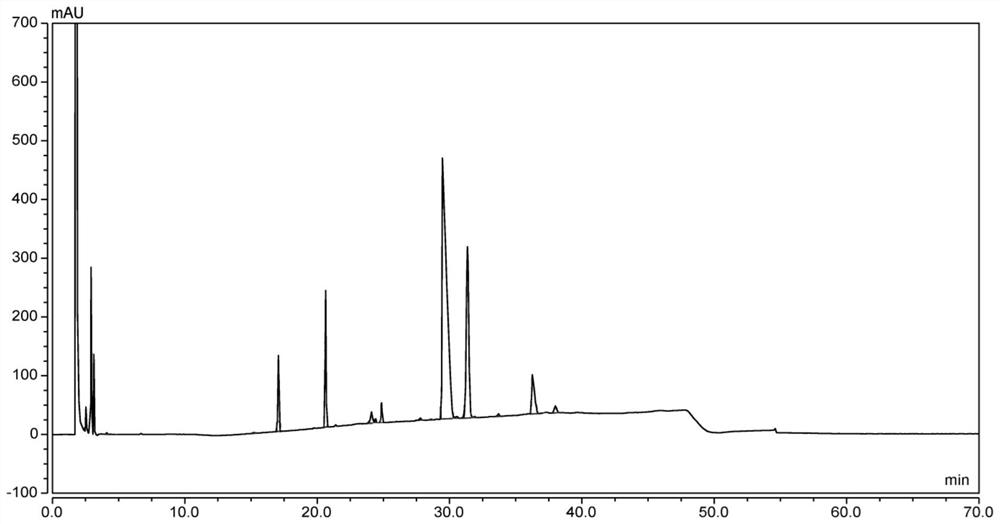
[0116] According to the same method as In Example 1, the above reaction liquid and the product DO3A-T-BU-ESTER were performed with high performance liquid chromatography (HPLC), the results are shown in Table 2.
[0117] Table 2, HPLC test results of Example 2 to 4
[0118]
[0119]
[0120]
[0121] The above results show that under the same other process conditions, the main product when using potassium carbonate is impurity 4 (tetraesteride product content is 73.10%), and there are more impurities 4 (tetraesteride produc...
Embodiment 5~7
[0123] The same content as Example 1 is no longer repeated, the difference is that in the step (1) of the DO3A-T-BU-ESTER preparation method, the solvent DMF is changed to ethanol, N, N- dimethylacetamide, acetonitrile (the amount of solvent is unchanged), the reaction liquid is obtained, and then subsequent separation and purification is carried out according to the same method to obtain the product DO3A-T-BU-ESTER.
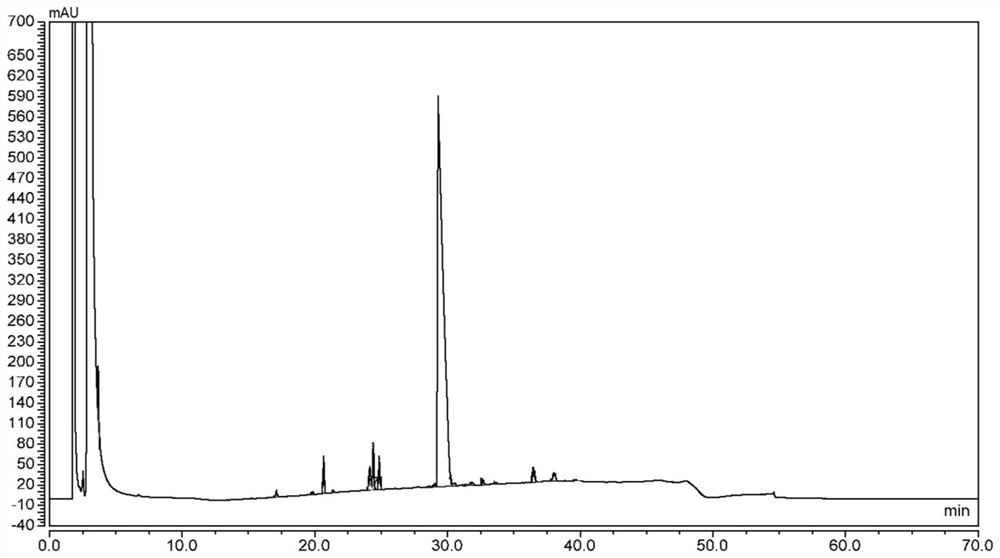
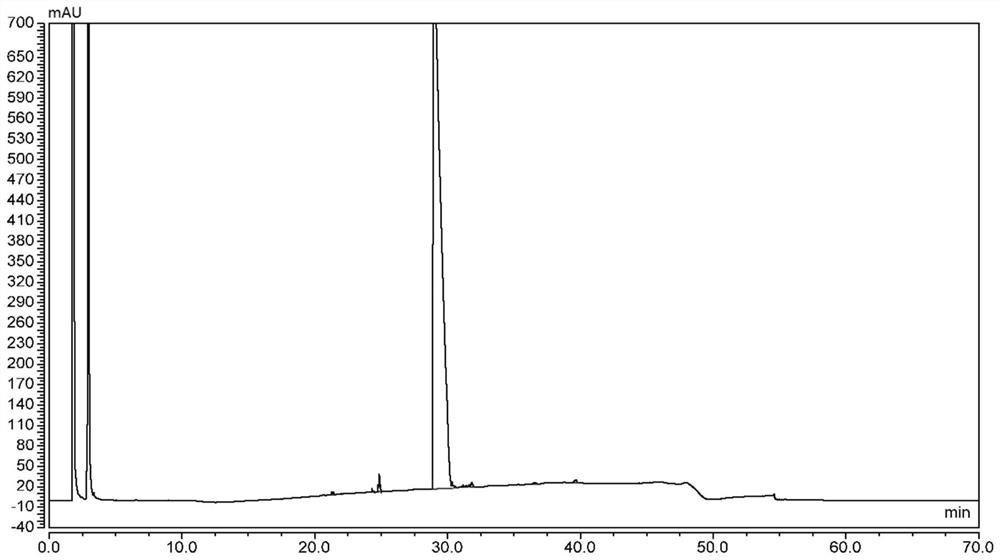
[0124] According to the same method as In Example 1, the above reaction liquid and the product DO3A-T-BU-ESTER were tested by high performance liquid chromatography (HPLC), and the results are seen separately Figures 3 to 9 and Tables 3 to 9.
[0125] Table 3, EXAMPLE 5 of the resulting reaction solution of hplc ( Figure 3 ) data
[0126]
[0127]Table 4, Example 5 of the product DO3A-T-BU-ESTER HPLC ( Figure 4 ) data
[0128]
[0129] Table 5, Example 6 of the resulting reaction solution of the HPLC ( Figure 5 ) data
[0130]
[0131] Table 6, Example 6 of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com