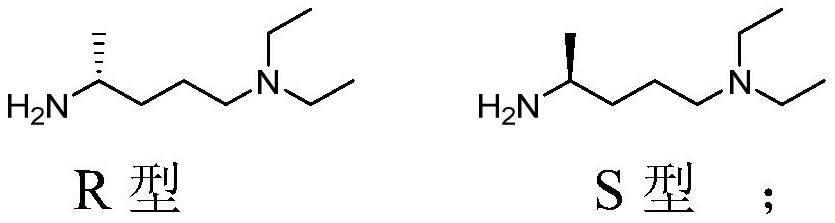Chiral amino quinazoline compound as well as preparation method and application thereof
A technology of aminoquinazoline and phenylquinazoline, which is applied in the field of chiral aminoquinazoline compounds and their preparation, can solve the problems of reduced drug efficacy, aggravated pollution, different biological activities and the like, and achieves a simple synthesis route Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
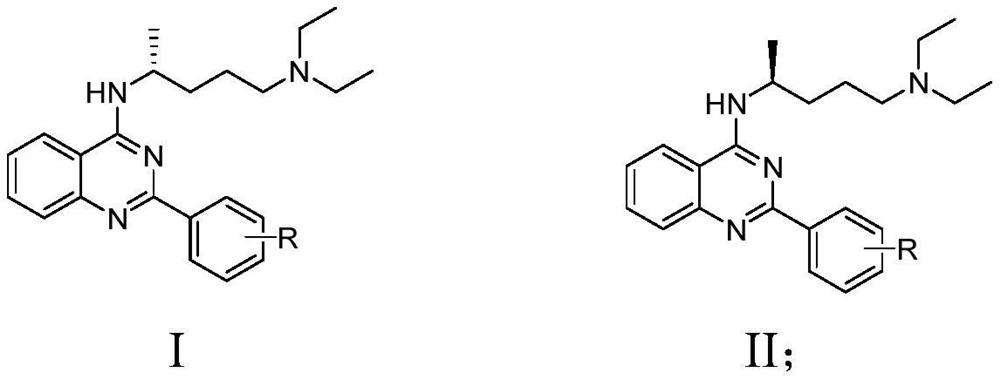
[0080] The preparation of embodiment 1 chiral amine
[0081] Dissolve 600g of S-mandelic acid in 1800ml of ethanol, then add 600g of 2-amino-5-diethylaminopentane, stir to release heat to room temperature, precipitate a large amount of solid, filter with suction, and retain the filtrate to obtain a white solid, 75 It was dried at °C to obtain 520 g of S-mandelate. The above mandelate was dissolved in 1200 mL of water, neutralized with 15% (w / v) sodium hydroxide solution to pH=11, stirred and extracted twice with 1000 mL of dichloromethane, and the filtrates were combined after separation, and the solution was mixed with waterNa 2 SO 4 Dry, filter and spin to dry to obtain (S)-2-amino-5-diethylaminopentane (ie (S)-N',N'-diethyl-1,4-pentanediamine), weighed 260g.
[0082] The filtrate obtained in the previous step was spin-dried and added with 1200 mL of water, neutralized to pH=11 with a 15% (w / v) sodium hydroxide solution, stirred and extracted with 1200 mL of dichlorometh...
Embodiment 2
[0083] The preparation of embodiment 2 (S)-4-amino-2-phenylquinazoline
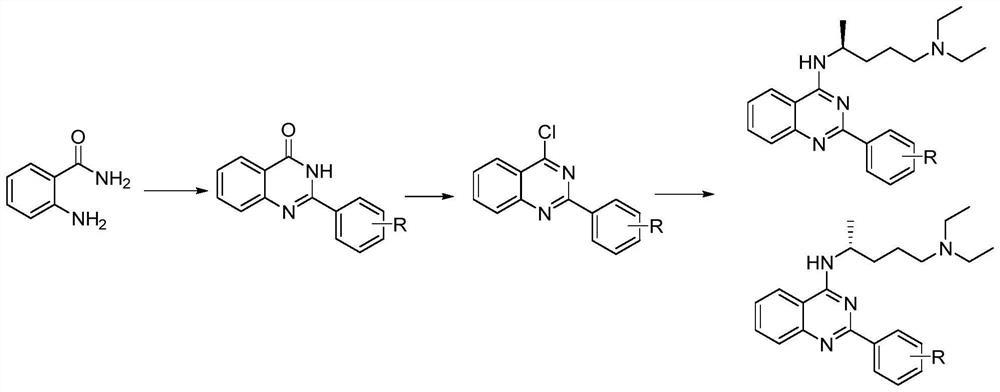
[0084] (1) Synthesis of 2-phenylquinazolin-4(3H)-one:
[0085] Take 50.0g anthranilamide, 40.0g benzaldehyde, 85g CuCl 2 , add 600 mL of ethanol, stir and react at 80 ° C for 16 h, TLC detection (developing solvent is ethyl acetate and petroleum ether mixed in a volume ratio of 1:1) After the anthranilamide reaction, add 500 mL of water to continue heating and stirring 1 h, filtered, and the filter cake was dried at 90° C. to obtain 67.2 g of a white solid. The solid was 2-phenylquinazolin-4(3H)-one, and the yield was 82.3%.
[0086] (2) Synthesis of 4-chloro-2-phenylquinazoline:
[0087] The solid obtained in step (1) was added to 200 mL of chloroform, 115.0 g of SOCl 2 , the reaction was carried out at 70°C for 12h, and the tail gas was absorbed by 30% (w / w) NaOH solution. TLC detection (developing solvent is obtained by mixing dichloromethane and petroleum ether in a volume ratio of 1:1) After the ...
Embodiment 3
[0094] Example 3 Preparation of (S)-4-amino-2-(4-chlorophenyl)-quinazoline
[0095] (1) Synthesis of 2-(4-chlorophenyl)-quinazolin-4(3H)-one:
[0096] Take 45.0g anthranilamide, 48.0g 4-chlorobenzaldehyde, 80.0g CuCl respectively 2 , add 500 mL of isopropanol, stir at 82 ° C for 16 h, TLC detection (developing solvent is ethyl acetate and petroleum ether mixed by volume 1:1) After the anthranilamide reaction, add 400 mL of water to continue heating Stir for 1 h, filter, and dry the filter cake at 90° C. to obtain 66.8 g of 2-(4-chlorophenyl)-quinazolin-4(3H)-one as a light yellow solid, with a yield of 81.5%.
[0097] (2) Synthesis of 4-chloro-2-(4-chlorophenyl)-quinazoline:
[0098] The solid obtained in step (1) was added to 200 mL of chloroform, 105 g of SOCl 2 , the reaction was carried out at 80°C for 12h, and the tail gas was absorbed by 30% (w / w) NaOH solution. TLC detection (developing solvent is obtained by mixing dichloromethane and petroleum ether in a volume ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com