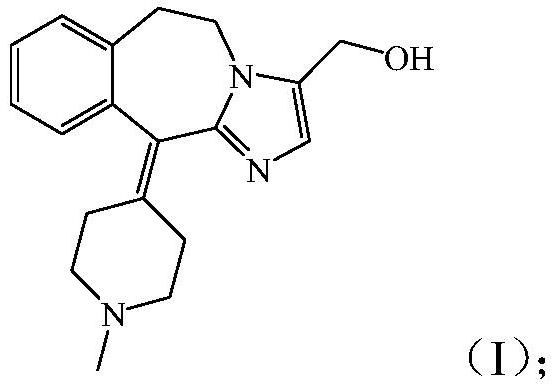
Preparation method of alcaftadine and purification method of intermediate of alcaftadine
A technology of alcaftadine and intermediates, which is applied in the field of preparation of high-content raw materials, can solve problems such as quality improvement, and achieve the effect of stable purity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The preparation method of the acarbazide product
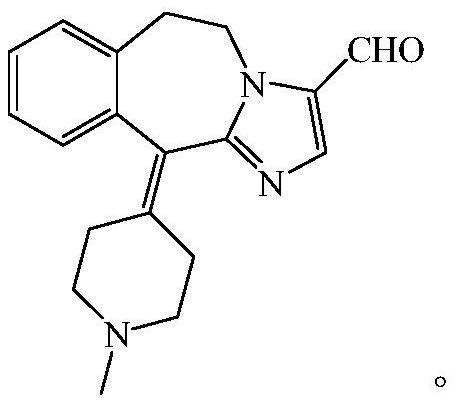
[0050] In the first aspect of the present invention, the present invention proposes a preparation method of acapatadine. According to an embodiment of the present invention, it is obtained by oxidizing AT3 through Dess-Martin reagent, and the oxidation reaction includes: combining AT3 and Part of the Dess-Martin reagent is oxidized, and after the oxidation reaction is carried out for a period of time, the remaining Dess-Martin reagent is added; preferably, the weight of the part of the Dess-Martin reagent is the total amount of the Dess-Martin reagent added 0.3 to 0.7 times,
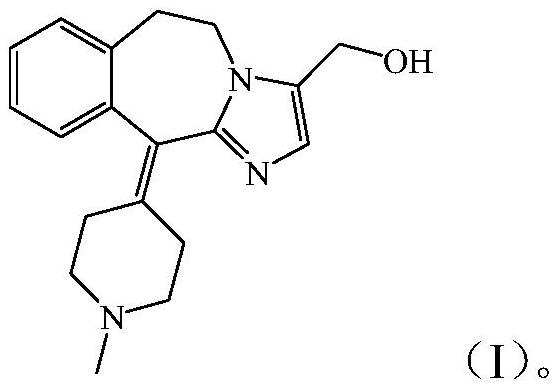
[0051] Wherein, described AT3 is the compound shown in formula (I):
[0052]
[0053] The oxidizing agent Dess-Martin reagent was added in portions.
[0054] According to the embodiment of the present invention, the quality of the additive is controlled to be 0.005 to 0.02 times the mass of AT3;
[0055] According to the embodiment of the pr...
Embodiment 1
[0083] AT3 is prepared from AT2, and its chemical formula is as follows:
[0084]
[0085] The concrete operation steps are as follows: add 2.2kg AT2, acetic acid (0.55kg), 37% formaldehyde solution (6L), and 1.25kg of anhydrous sodium acetate into the reactor, heat to 80~100° C., stir for 18~30 hours, The reaction mixture was cooled to 25-30°C. Dichloromethane (22 L) was added to the reaction mixture and stirred for 30 minutes. The pH of the aqueous layer was adjusted to 8-11 with 20% sodium hydroxide. Stirring was continued for 0.5 hour, the organic layer was separated, the organic layer was washed twice with 20% sodium hydroxide solution, stirred evenly with anhydrous sodium sulfate, and left to dry. Suction filtration, the gained filtrate is concentrated under reduced pressure at 35~45 ℃ until there is solid precipitation, suction filtration, the obtained filter cake is the crude product containing AT3, in the crude product containing AT3, AT3 purity is 77.98%, AT2 re...
Embodiment 2
[0087] The general idea of the following experimental group and comparative group is: carry out slurry washing treatment on the crude product containing AT3 prepared in Example 1, so as to obtain purified AT3.
[0088] Experimental group 1
[0089] The crude product (AT3 content 77.98%, AT2 residual 19.16%, impurity C content is 2.86%) 20g containing AT3 of embodiment 1 gained, the methylene chloride of 80g, the pH of 320g are that the potassium carbonate aqueous solution of 10 is added in the 1L there-necked flask , heated to 30°C, stirred for 1 hour, filtered while hot, and dried the filter cake to obtain purified AT3 with a yield of 96.1%. In the purified AT3, the content of AT3 was 98.98%, the residual AT2 was 0.74%, and the content of impurity C was 0.28%.
[0090] Experimental group 2
[0091] The crude product containing AT3 obtained in Example 1 (AT3 content 77..98%, AT2 residual 19.16%, impurity C content 2.86%) 20g, 60g of dichloromethane, 420g of pH 8 sodium bic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com