Method for preparing two-dimensional cross-linked polymer
A technology for cross-linking polymers and polymers, applied in the field of polymer chemistry, can solve the problems of dynamic instability, the difficulty of interfacial polymerization to prepare two-dimensional polymers on a large scale, and the inability to maintain two-dimensional structures.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 1.1 The preparation method of the amphiphilic monofunctional monomer A specifically includes the following steps:
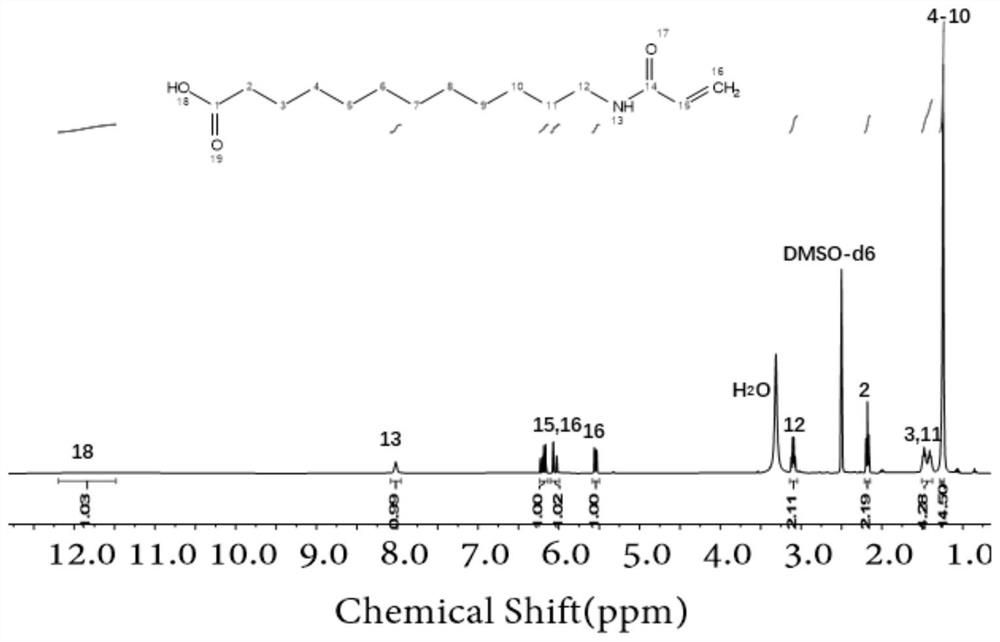
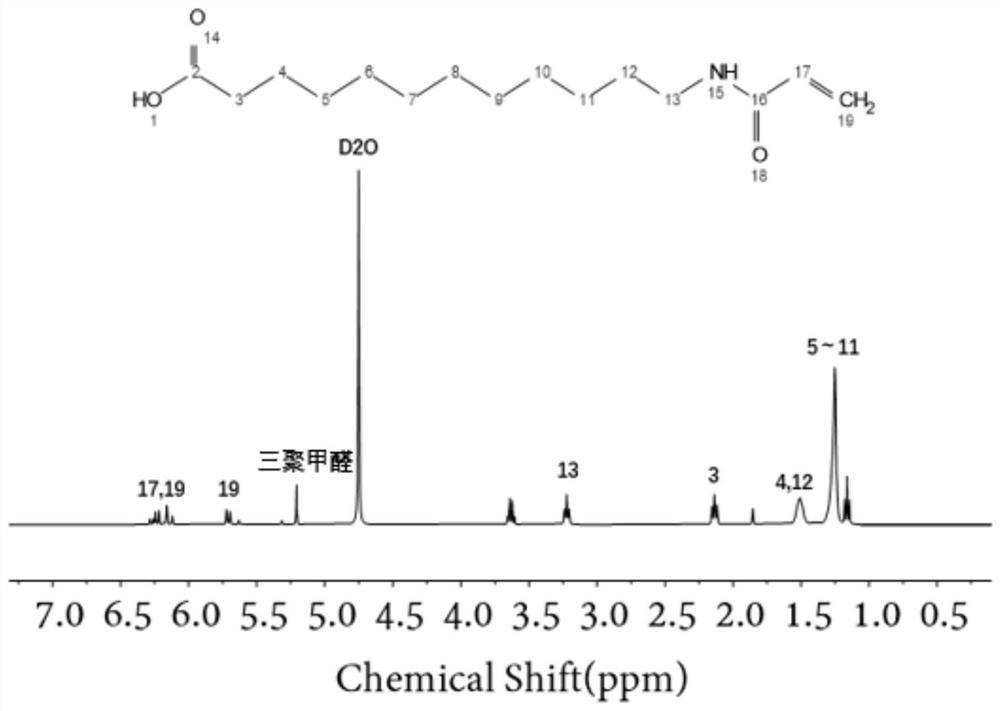
[0055] 50 mL of ethanol, 5 mL of deionized water and sodium hydroxide (1.11 g, 27.86 mmol) were added to 12-aminododecanoic acid (2 g, 9.29 mmol), and acryloyl chloride (1 mL, 12 mmol) was added dropwise under an ice-water bath, and the dropwise addition was completed. Then, the ice-water bath was removed, and then the reaction was performed at room temperature for 3 hours. After the reaction was completed, suction filtration was performed, and the filtrate was acidified with hydrochloric acid. Then, 500 mL of deionized water was added. At this time, a white solid was precipitated. Body A in 70% yield. like figure 1 shown, the monofunctional monomer A was characterized by NMR.
[0056] The synthetic route of monofunctional monomer A is shown below:
[0057]
[0058] 1.2 Self-assembly of the amphiphilic monofunctional monomer A, which specifically inc...
Embodiment 2
[0064] The preparation method of amphiphilic monofunctional monomer B specifically comprises the following steps:
[0065] To a 100 mL round-bottomed flask was added p-aminobenzoic acid (2.0 g, 14.58 mmol), 10 mL of dichloromethane, triethylamine (6 mL, 43.28 mmol), and acryloyl chloride (1.78 mL, 21.9 mmol) was added dropwise under an ice bath. After dropping, the ice bath was removed, and the reaction was kept overnight at room temperature. After the reaction, 1 mol / L hydrochloric acid was directly used for acidification, and a solid was precipitated. Finally, suction filtration and washing were performed to obtain monofunctional monomer B with a yield of 74%. The H NMR spectrum of the obtained amphiphilic monofunctional monomer B is as follows: Figure 10 shown.
[0066] The synthetic route of monofunctional monomer B is shown below:
[0067]
[0068] To the monofunctional monomer B (1.00 g, 5.23 mmol), add 10 mL of deionized water, sonicate for 5 minutes, then add 1.2...
Embodiment 3
[0071] According to the steps of Example 2, the (cross-linking agent) ethylene glycol dimethacrylate was changed into (cross-linking agent) divinylbenzene (2.1 μL, 14.8 μmol), and the two-dimensional supramolecules were obtained by shaking uniformly;
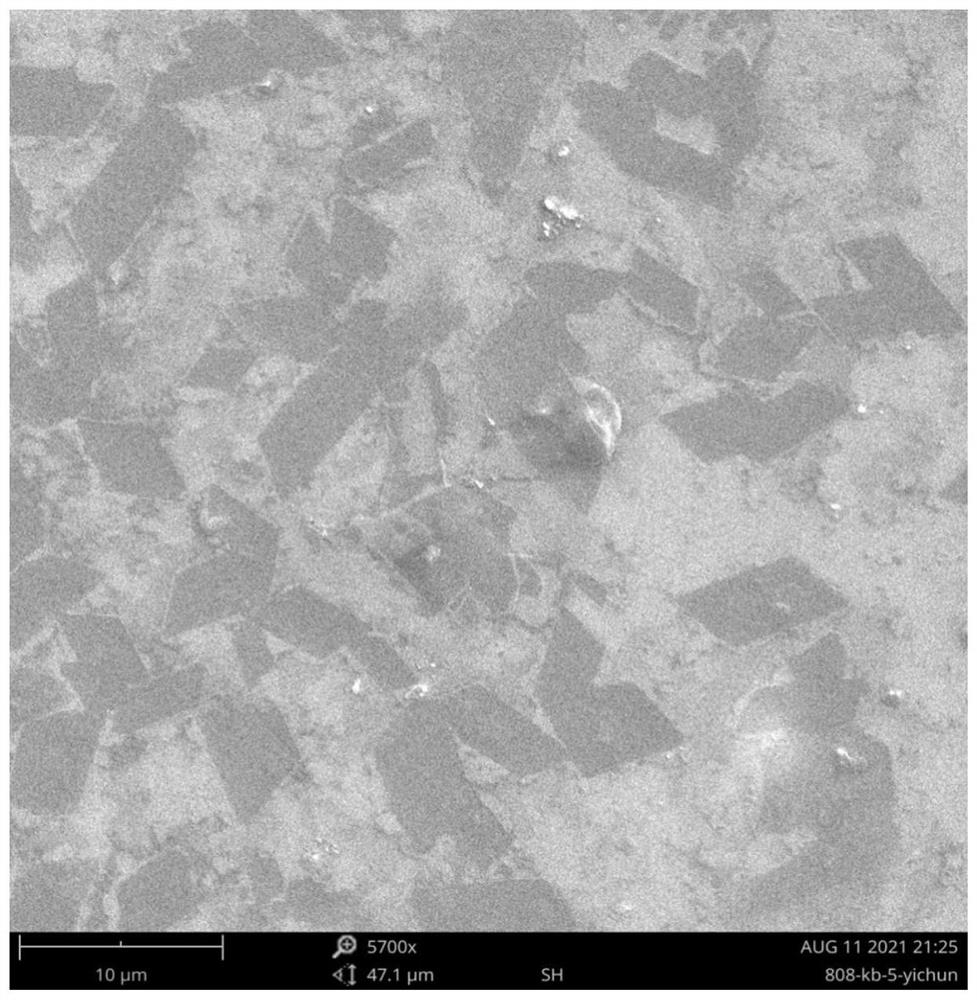
[0072] Add potassium persulfate (4 mg, 14.86 μmol) aqueous solution to the two-dimensional supramolecular solution, and then add sodium thiosulfate pentahydrate (3.34 mg, 13.45 μmol) aqueous solution after deoxygenation and nitrogen operation, and then polymerize at 30 °C for 12 After the polymerization was completed, dialysis was performed with a dialysis bag with a molecular weight cut-off of 8,000-14,000 for 24 hours, and the water was changed every 3 hours for a total of 4 times to obtain a two-dimensional polymer. like Figure 12 As shown, the morphology of the polymer was characterized by optical microscopy (OM), and a large number of lamellar structures were observed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com