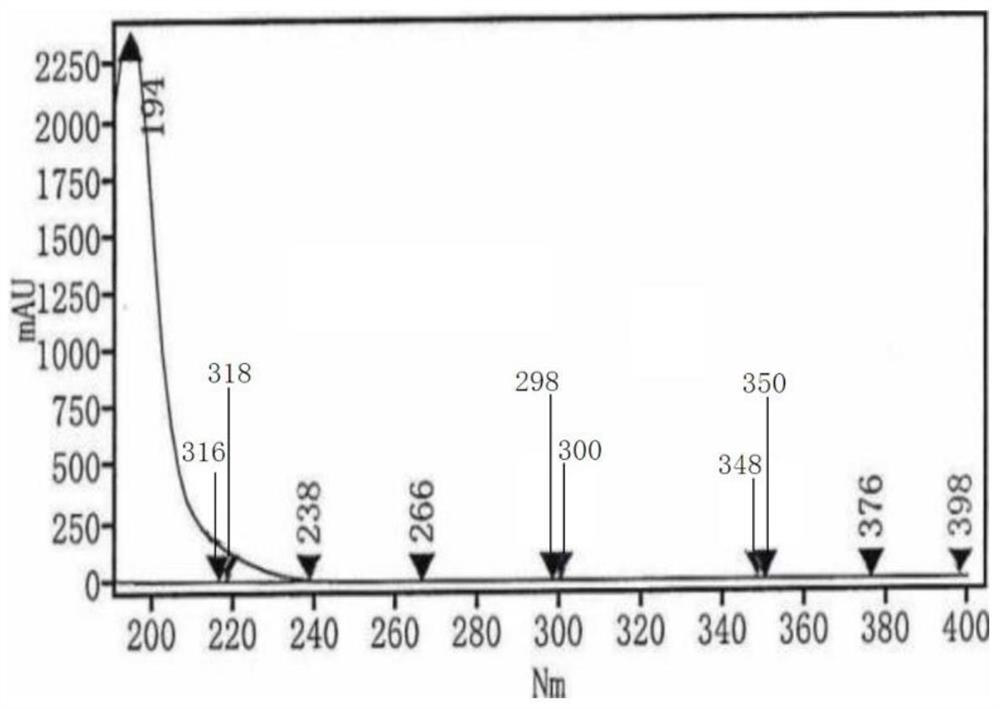
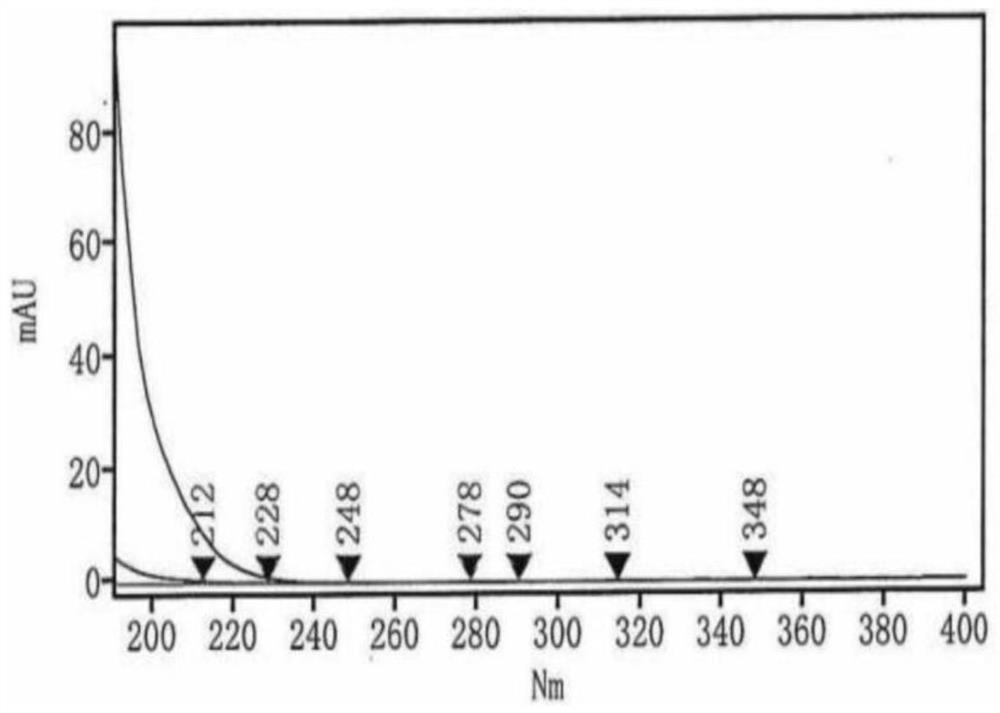
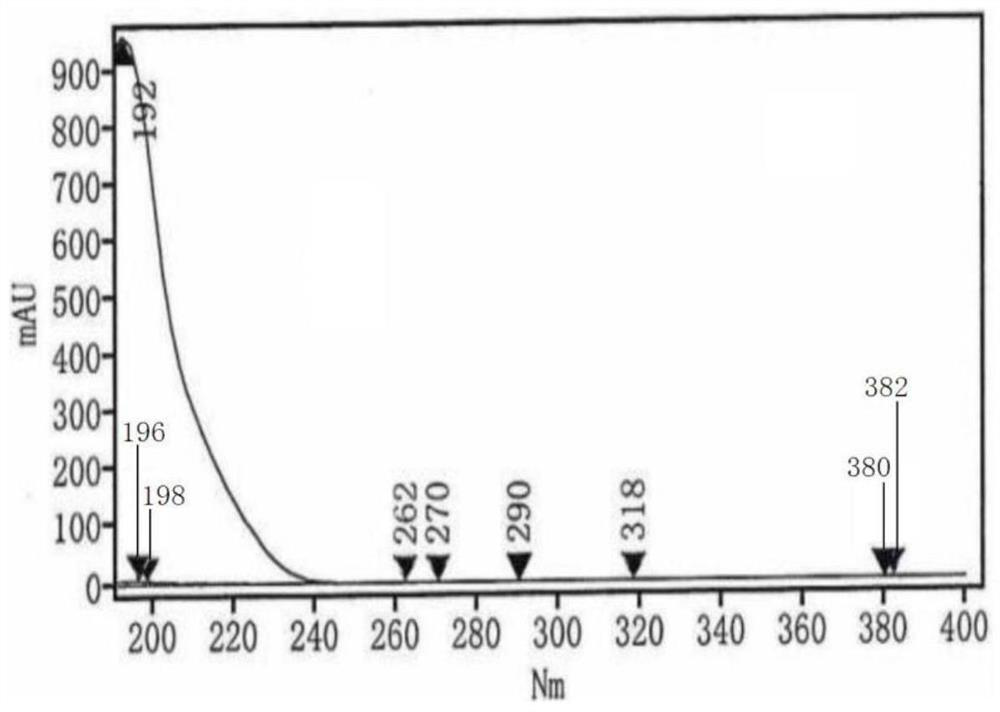
HPLC (High Performance Liquid Chromatography) test method for related substances in L-prolinamide
A technology related to substances, prolineamides, applied in the field of column chromatography testing or analyzing materials, can solve the problems of short retention time, ineffective detection, small response value, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Instruments and reagents
[0035] ① Instrument: High performance liquid chromatography, Agilent 1260; electronic balance, Mettler XSR 105; pH meter, Mettler FE28.
[0036] ②Reagents: sodium octanesulfonate (chromatographic grade), Scharlau; sodium dihydrogen phosphate dihydrate (analytical grade), Sinopharm Group; acetonitrile (chromatographic grade); phosphoric acid (analytical grade), Sinopharm Group.
[0037] ③Chromatographic conditions: chromatographic column Agilent TC-C18(2), 5μm; 4.6×250mm, flow rate 0.5ml / min; detection wavelength 200nm; injection volume 10μL; column temperature 30°C.
[0038] ④ Mobile phase: Weigh 5.13 g of sodium octane sulfonate and 1.48 g of sodium dihydrogen phosphate dihydrate, add 850 ml of water to dissolve, add 150 ml of acetonitrile and mix, and adjust the pH to 3.0 with phosphoric acid.
[0039] ⑤ Solvent: mobile phase.
[0040] (2) Preparation of each solvent
[0041] ① Each impurity positioning solution: Weigh the appropriate...
Embodiment 2
[0063] The chromatographic conditions in this example: the flow rate is 0.4 ml / min, and other chromatographic conditions are the same as those in Example 1.
[0064] Take 10 μL of the system suitability solution, inject it into the liquid chromatograph, and record the chromatogram.
[0065] The detection results of related substances in the system suitability solution are shown in Table 2, and the obtained HPLC chromatogram is shown in Table 2. Figure 7 , it can be seen from Table 2 that all impurity peaks are effectively separated, and the main peak is completely separated from the adjacent impurity peaks.
[0066] Table 2: System Suitability Solution Related Substance Test Results
[0067] Impurity name Retention time (min) degree of separation Number of theoretical plates L-Hydroxyproline 8.292 1.7 28718 L-Proline 9.665 1.96 17762 Proline dimer 11.089 4.96 23867 L-Prolineamide 28.625 16.4 17367
Embodiment 3
[0069] The chromatographic conditions in this example: the flow rate is 0.6 ml / min, and other chromatographic conditions are the same as those in Example 1.
[0070] Take 10 μL of the system suitability solution, inject it into the liquid chromatograph, and record the chromatogram.
[0071] The detection results of related substances in the system suitability solution are shown in Table 3, and the obtained HPLC chromatogram is shown in Table 3. Figure 8 , it can be seen from Table 3 that all impurity peaks are effectively separated, and the main peak is completely separated from the adjacent impurity peaks.
[0072] Table 3: System Suitability Solution Related Substance Test Results
[0073] Impurity name Retention time (min) degree of separation Number of theoretical plates L-Hydroxyproline 5.502 1.71 22619 L-Proline 6.407 1.91 18082 Proline dimer 7.384 5 21316 L-Prolineamide 18.907 16.96 18078
PUM
| Property | Measurement | Unit |
|---|---|---|
| Linear | aaaaa | aaaaa |
| Linear | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com