Preparation method of high-purity L-nicotine medical intermediate
A high-purity, intermediate technology, applied in the direction of organic chemistry, can solve the problems of difficult recovery, long reaction time, increased side reactions, etc., to avoid the use of organic solvents, shorten the reaction time, and reduce the cost of raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] A preparation method of a high-purity L-nicotine pharmaceutical intermediate, the steps are as follows:
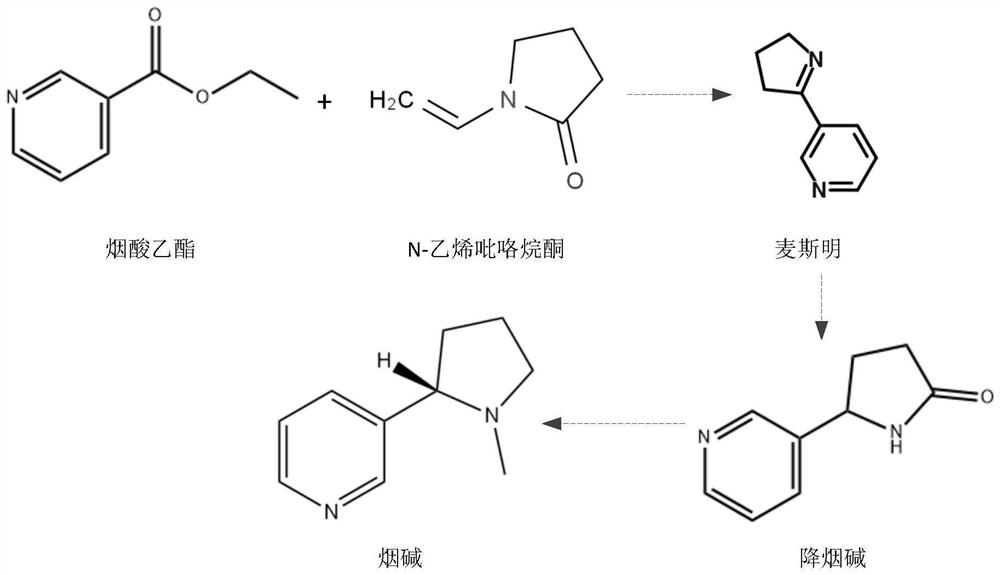
[0060] (1) Synthesis of Mesmin
[0061] 1500L of dry xylene was pumped into the 5000L condensation reaction kettle, after nitrogen replacement, 56kg of sodium metal and 14kg of tert-butanol were added, the temperature was raised to 84°C and kept for two hours, and then cooled to 45°C for subsequent use. 1000L of xylene, 302kg of ethyl nicotinate and 245kg of N-vinylpyrrolidone were pumped into a 3000L preparation reaction kettle and kept at 30°C, stirred and dissolved, and then poured into a high position.
[0062] Controlled at 45 ° C, the high-level reaction was dropped into the condensation reaction kettle, and the gas was released during the period. After the dropwise addition was completed, the temperature was raised to 80° C. for 5 h, and the reaction of ethyl nicotinate was detected by TCL. Cool down to below 20°C for later use.
[0063] 1000kg of concentr...
Embodiment 2
[0069] A preparation method of a high-purity L-nicotine pharmaceutical intermediate, the steps are as follows:
[0070] (1) Synthesis of Mesmin
[0071] 1500L of dry xylene was pumped into the 5000L condensation reaction kettle, after nitrogen replacement, 28kg of sodium metal and 7kg of tert-butanol were added, the temperature was raised to 84°C and kept for two hours, and then cooled to 45°C for subsequent use. 500L of xylene, 151kg of nicotinic acid ethyl ester and 123kg of N-vinylpyrrolidone were pumped into the 3000L preparation reaction kettle and kept at 30°C, stirred and dissolved, and put into a high position.
[0072] Controlled at 50 °C, the high-level reaction was dropped into the condensation reaction kettle, and the gas was released during the period. After the dropwise addition, the temperature was raised to 75° C. for 3 hours, and the reaction of ethyl nicotinate was detected by TCL. Cool down to below 20°C for later use.
[0073] Put 500kg of concentrated h...
Embodiment 3
[0078] A preparation method of a high-purity L-nicotine pharmaceutical intermediate, the steps are as follows:
[0079] (1) Synthesis of Mesmin
[0080] 1500L of dry xylene was pumped into the 5000L condensation reaction kettle. After nitrogen replacement, 84kg of metallic sodium and 21kg of tert-butanol were added, the temperature was raised to 84°C and kept for 2h, and then cooled to 45°C for use. 2000L of xylene, 454kg of nicotinic acid ethyl ester and 372kg of N-vinylpyrrolidone were pumped into the 3000L preparation reaction kettle, kept at 30°C, stirred and dissolved, and put into a high position.
[0081] Controlled at 50 °C, the high-level reaction was dropped into the condensation reaction kettle, and the gas was released during the period. After the dropwise addition was completed, the temperature was raised to 75° C. for 4 hours, and the reaction of ethyl nicotinate was detected by TCL. Cool down to below 20°C for later use.
[0082] Put 1500kg of concentrated hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com