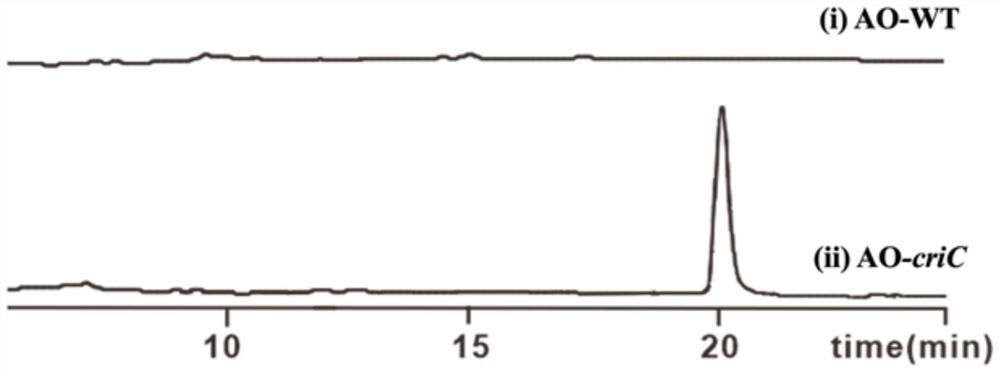
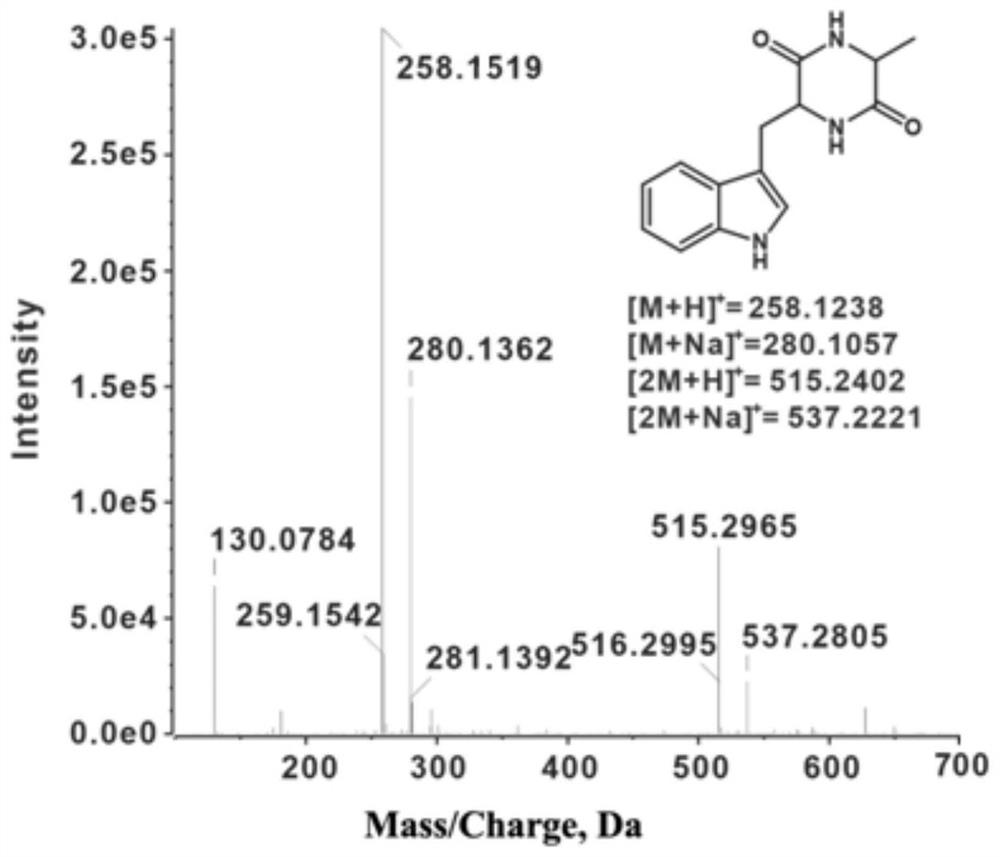
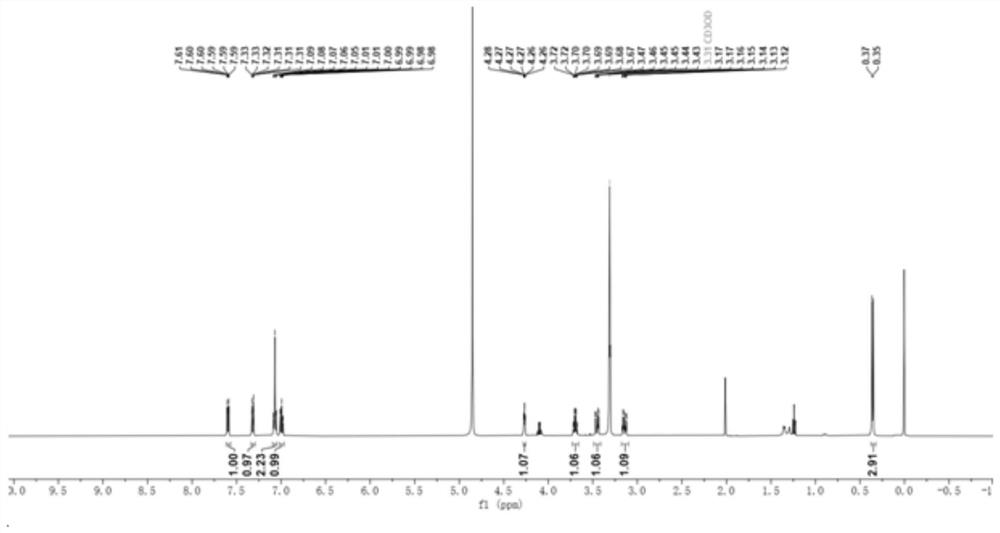
Method for preparing L-tryptophan-L-alanine cyclic dipeptide by using aspergillus oryzae
A tryptophan and alanine technology, applied in the field of biomedicine, can solve the problems of difficult chemical synthesis, unstable fermentation, high price, etc., and achieve the effects of simple and easy separation and purification, breaking through resource limitations, and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0027] Embodiment 1: In this embodiment, a method for preparing L-tryptophan-L-alanine cyclic dipeptide using Aspergillus oryzae is carried out according to the following steps:
[0028] Step S1: construction of expression plasmid pMA-criC;
[0029] Using the genomic DNA of Eurotium cristatum as the template, using P1F as the forward primer and P1R as the reverse primer, the fragment 1 of the cyclic dipeptide synthase encoding gene criC was obtained by amplification, and then P2F was used as the forward primer. The primer and P2R are reverse primers, and the second fragment of the cyclic dipeptide synthase encoding gene criC is obtained by amplification; then the genomic DNA of Eurotium cristatum is used as the template, and the pMA plasmid is cut and linearized with KpnI enzyme. The fragment is a vector, and the fragment 1 and fragment 2 of the cyclic dipeptide synthase encoding gene criC are inserted into the restriction site KpnI of the pMA plasmid to obtain the expression ...
specific Embodiment approach 2
[0036] Specific embodiment 2: The difference between this embodiment and specific embodiment 1 is: in step S1, fragment 1 and fragment 2 of the cyclic dipeptide synthase encoding gene criC and the pMA plasmid fragment that has been linearized by KpnI digestion are according to 1: After the ligation was completed, the ligation system was transformed into Escherichia coli DH5α, and positive clones were obtained after overnight culture. Finally, after PCR verification and enzyme digestion verification, the expression plasmid was obtained. pMA-criC.
[0037] Other steps are the same as in the first embodiment.
specific Embodiment approach 3
[0038] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is: in step S2, the step of culturing a high-yielding strain based on the L-tryptophan-L-alanine cyclic dipeptide expressing the plasmid pMA-criC As follows: take the spore preservation solution of Aspergillus oryzae and insert it into the DPY medium containing 100 mL, and at 28-30° C., shake and culture at 180-200 rpm for 2-3 days to obtain Aspergillus oryzae. ) of the thalli; the thalli of Aspergillus oryzae were collected by filtration and washed with sterile water for 3 to 5 times, and then 20 mL of cell wall lysing solution was added, and at a temperature of 25 to 28 ° C, gently shake for 2 to 3 hours to obtain protoplasts; wash the protoplasts twice with sterilized 0.8M NaCl solution, add buffer II and buffer III, and then add 10 μg of the expression plasmid pMA-criC, after mixing, ice bath for 18-20 min, and then add Add 1 mL of buffer III to the mixed system, incubate at room temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com