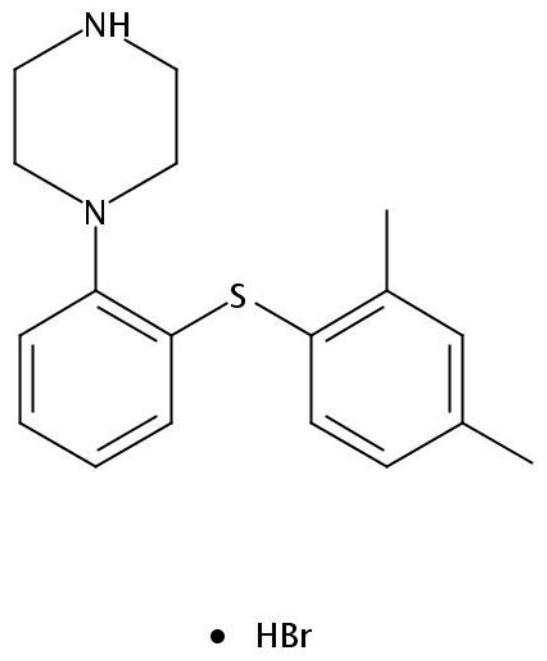
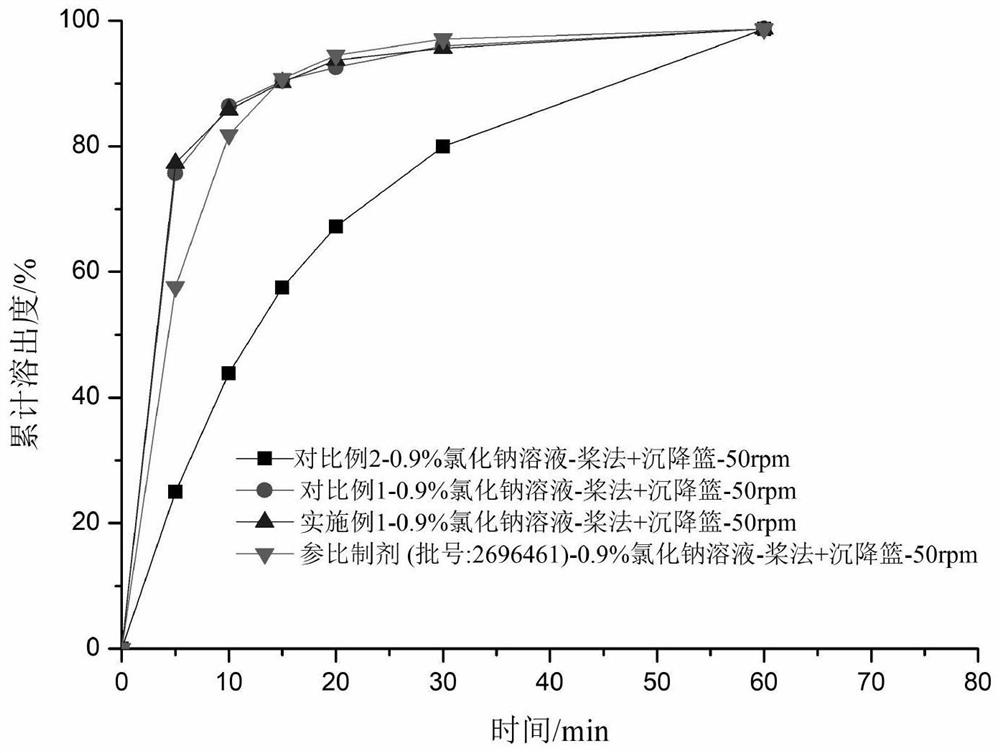
Hydrobromic acid vortioxetine oral soluble film agent and preparation method thereof
A technology of vortioxetine and oral fast-dissolving film agent, which is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Due to the limited drug loading and other problems, the effect of reducing the risk of air retention, reducing the types of excipients, and saving material costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
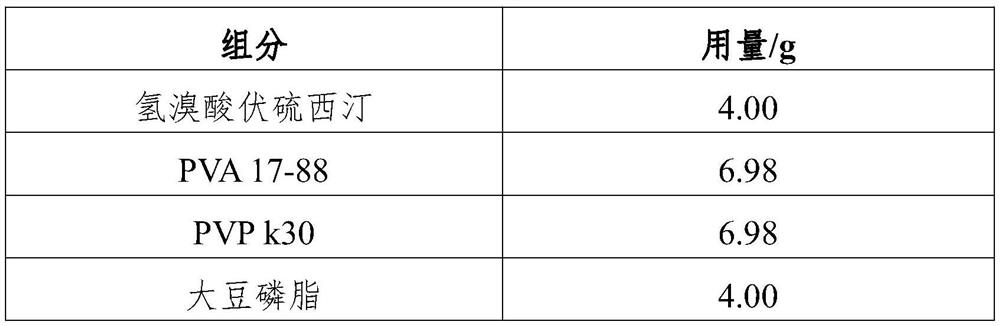
[0047] The vortioxetine hydrobromide oral solvent film is prepared from the component of the corresponding weight of table 1:
[0048] Table 1
[0049] component Dosage / g vortioxetine hydrobromide 9.08 Povidone (PVP-VA64) 18.64 Polyoxyethylene (PEO) 12.43 polyethylene glycol 5.00 malic acid 3.00 stevioside 0.35 Sodium chloride 1.50
[0050] The preparation method is as follows:
[0051] S1 takes by weighing the corresponding weights of PVP-VA64, PEO, vortioxetine hydrobromide, polyethylene glycol, malic acid, stevioside and sodium chloride in Table 1, and mixes to obtain a mixture;
[0052] S2 Add the mixture into the hot melt extruder, and send it to the hot melt zone through the feed zone of the hot melt film press. The raw materials are gradually melted and mixed, and the melted mixture is continuously output through the metering zone. The extrusion temperature is set to 80°C, 120°C, 140°C, 140°C, 140°C, 140°C, 140...
Embodiment 2
[0056] The vortioxetine hydrobromide oral solvent film is prepared from 2 components by corresponding weight:
[0057] Table 2
[0058] component Dosage / g vortioxetine hydrobromide 38 Povidone (PVP-VA64) 42 Polyoxyethylene (PEO) 48 polyethylene glycol 15 malic acid 5 stevioside 5 Sodium chloride 3.5
[0059] The preparation method is as follows:
[0060] S1 takes by weighing the corresponding weights of PVP-VA64, PEO, vortioxetine hydrobromide, polyethylene glycol, malic acid, stevioside and sodium chloride in Table 1, and mixes to obtain a mixture;
[0061] S2 Add the mixture into the hot melt extruder, and send it to the hot melt zone through the feed zone of the hot melt film press. The raw materials are gradually melted and mixed, and the melted mixture is continuously output through the metering zone. The extrusion temperature is set to 80°C, 120°C, 140°C, 140°C, 140°C, 140°C, 140°C, 140°C, the melt temperature i...
Embodiment 3
[0065] The vortioxetine hydrobromide oral solvent film is prepared from the components of table 3 by weight:
[0066] table 3
[0067] component Dosage / g vortioxetine hydrobromide 2 Povidone (PVP-VA64) 10 Polyoxyethylene (PEO) 10 polyethylene glycol 2 malic acid 3.00
[0068] The preparation method is as follows:
[0069] S1 takes by weighing PVP-VA64, PEO, vortioxetine hydrobromide, polyethylene glycol and malic acid of corresponding weight in table 1, and obtains mixture after mixing;
[0070] S2 Add the mixture into the hot-melt extruder, and send it to the hot-melt zone through the feed zone of the hot-melt film press. The raw materials are gradually melted and mixed, and the melted mixture is continuously output through the metering zone; set the extrusion temperature 80°C, 120°C, 140°C, 140°C, 140°C, 140°C, 140°C, 140°C, melt temperature 137°C, rotation speed 20 rpm;
[0071] The melted mixture output from S3 is passed thr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com