Preparation method of vitamin A acetate
A technology of acetate and vitamins, applied in the direction of organic chemistry, etc., can solve the problems of low yield and high process safety risk, and achieve the effect of simplifying the reaction route, improving safety, and eliminating potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
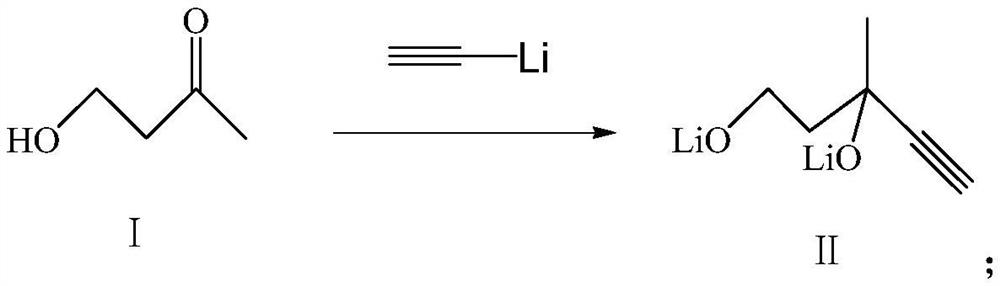
[0038] (1) Synthesis of compound (II)
[0039] The ether solution of ethynyl lithium (100 mL, 0.5 mol of ethynyl lithium) was pre-cooled to -15 °C, and the ether solution of 4-hydroxy-2-butanone (50 mL, 0.2 mol of 4-hydroxy-2-butanone) was slowly added dropwise. , 0.5h was added dropwise, and the reaction was continued at -15~-10℃ for 1h.
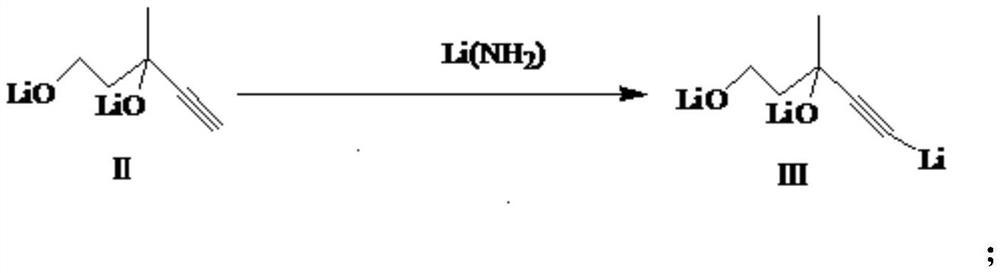
[0040] (2) Synthesis of compound (III)
[0041] The solid lithium amide (0.25mol) was put into the reaction solution obtained in step (1), and the reaction was stirred at -15 to -10° C. for 1.5 h. After the reaction was completed, 3-methyl-4-pentyne-1,3-diol ether solution (25mL, 0.07mol diol) was slowly added dropwise, the dropwise addition was completed in 0.5h, and the reaction was continued at -15~-10°C for 1.5 hours. h.
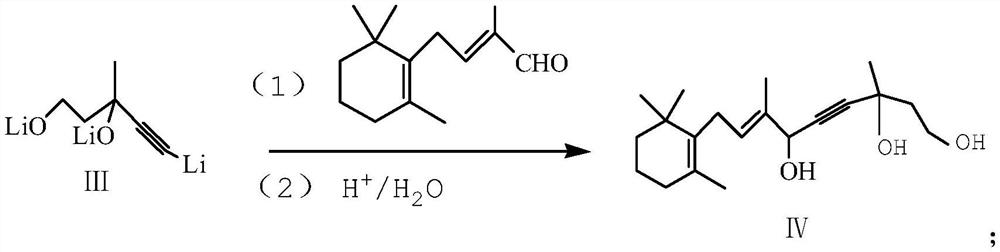
[0042] (3) Synthesis of compound (IV)
[0043] 2-Methyl-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-butene-1-aldehyde (0.2mol) was slowly added dropwise to step (2) In the obtained reaction solution, the dropwise add...
Embodiment 2
[0054] (1) Synthesis of compound (II)
[0055] The isopropyl ether solution of ethynyl lithium (100 mL, 0.5 mol ethynyl lithium) was pre-cooled to -10 °C, and 4-hydroxy-2-butanone isopropyl ether solution (50 mL, 0.2 mol of 4-hydroxy-2-butanone) was slowly added dropwise. -butanone), the dropwise addition was completed for 1 h, and the reaction was continued at -15 to -10 °C for 1.5 h.
[0056] (2) Synthesis of compound (III)
[0057] The solid lithium amide (0.25mol) was put into the reaction solution obtained in step (1), and the reaction was stirred at -15 to -10° C. for 1.5 h. After the reaction was completed, 3-methyl-4-pentyne-1,3-diol isopropyl ether solution (25 mL, 0.08 mol of diol) was slowly added dropwise, the dropwise addition was completed for 1 h, and the reaction was continued at -10 to -5 °C. 2h.
[0058] (3) Synthesis of compound (IV)
[0059] 2-Methyl-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-butene-1-aldehyde (0.2mol) was slowly added dropwise to step (2)...
Embodiment 3
[0070] (1) Synthesis of compound (II)
[0071] The isopropyl ether solution of ethynyl lithium (100 mL, 0.5 mol ethynyl lithium) was pre-cooled to -10 °C, and 4-hydroxy-2-butanone isopropyl ether solution (50 mL, 0.2 mol of 4-hydroxy-2-butanone) was slowly added dropwise. -butanone), the dropwise addition was completed for 1 h, and the reaction was continued at -15 to -10 °C for 1.5 h.
[0072] (2) Synthesis of compound (III)
[0073] The solid lithium amide (0.25mol) was put into the reaction solution obtained in step (1), and the reaction was stirred at -15 to -10° C. for 1.5 h. After the reaction was completed, 3-methyl-4-pentyne-1,3-diol isopropyl ether solution (25 mL, 0.08 mol of diol) was slowly added dropwise, the dropwise addition was completed for 1 h, and the reaction was continued at -10 to -5 °C. 2h.
[0074] (3) Synthesis of compound (IV)
[0075] 2-Methyl-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-butene-1-aldehyde (0.2mol) was slowly added dropwise to step (2)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com