Method for continuously synthesizing 9-phenol phenanthrene compound under promotion of visible light
A visible light and compound technology, applied in the preparation of organic compounds, chemical instruments and methods, formation/introduction of hydroxyl groups, etc., can solve the problems of harsh reaction conditions, long reaction time, high cost, etc., and achieves simple operation and reaction time. The effect of shortening and increasing conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of Example 1 Compound 2a:
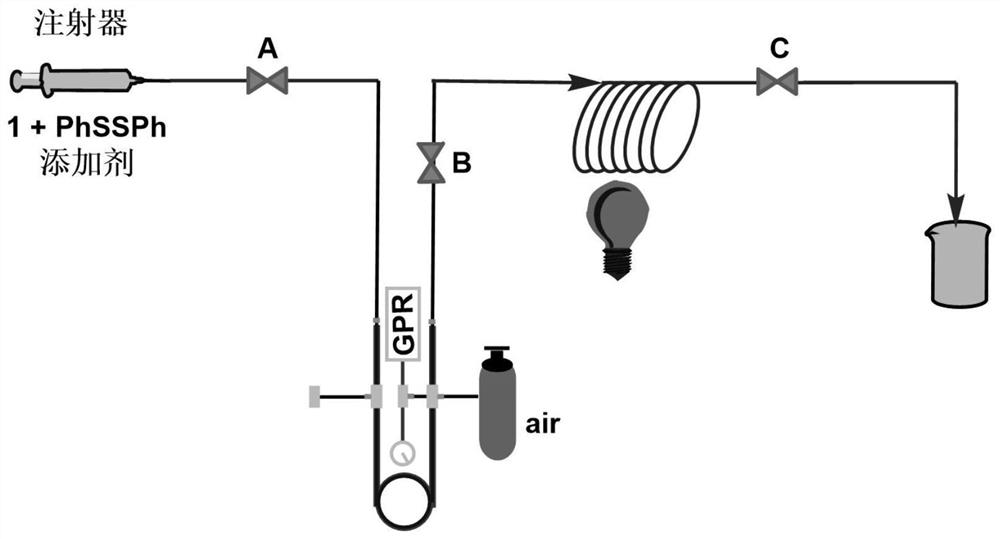
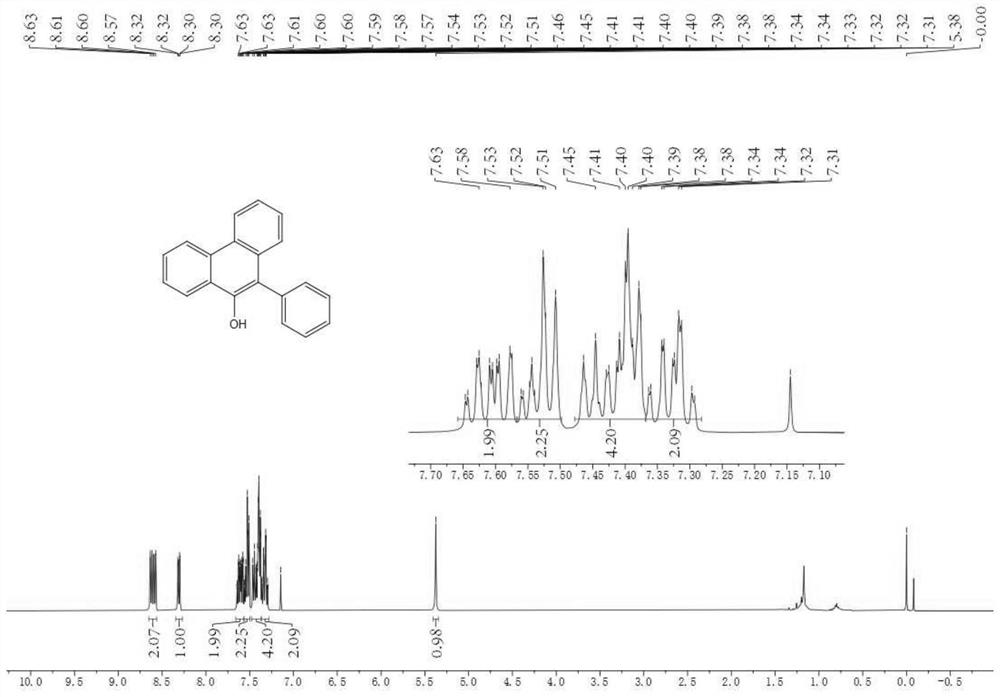
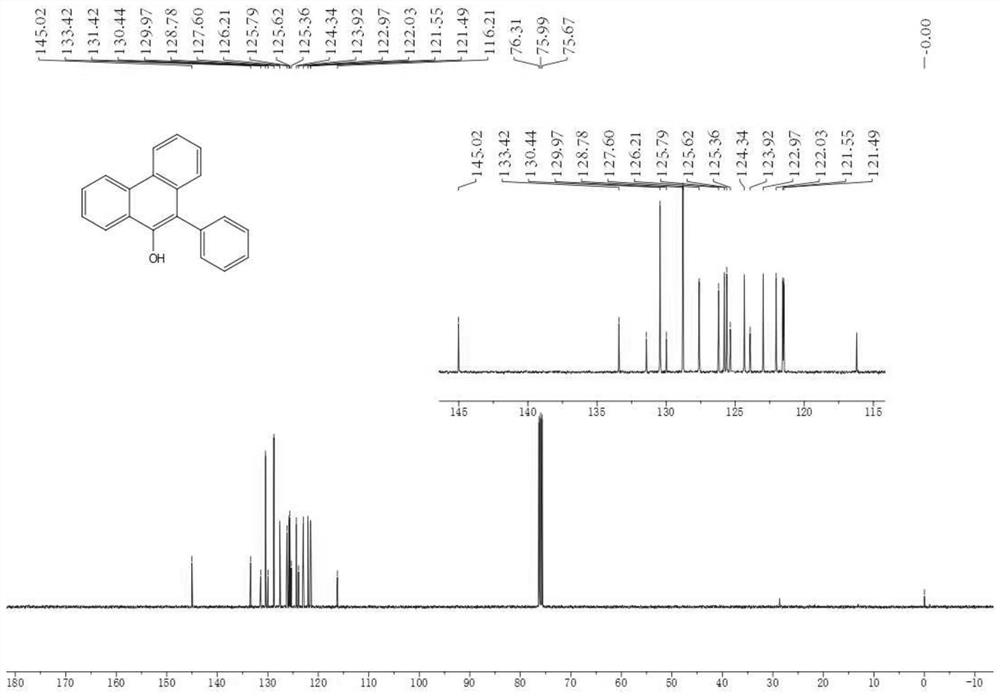
[0039] 0.3 mmol (0.0763 g) of compound 1a, 0.3 mmol (0.0655 g) of diphenyl disulfide and 0.6 mmol (0.0360 g) of acetic acid were dissolved in 3 mL of acetonitrile to obtain a homogeneous solution, which was added to a syringe; the syringe was set to inject pump, and inject the mixed solution in the syringe at a flow rate of 0.3mL / min, and then uniformly mix with air through the gas pressure regulating valve and flow into the microchannel reaction device; place the microchannel reaction device in a 30W blue LED lamp Irradiation; the reaction volume of the microchannel reaction device is V=3mL, and the reaction time is 10min; after the reaction in the microchannel reactor goes through one cycle, the reaction liquid is collected, and the product yield calculated by HPLC is 81%, and the volume ratio is 2:1 Extract with ethyl acetate / saturated aqueous sodium chloride solution (V=10 mL×3), and after concentration, the product 2a is obtain...
Embodiment 2
[0040] Synthesis of Example 2 Compound 2a:
[0041] 0.3 mmol (0.0763 g) of compound 1a, 0.3 mmol (0.0655 g) of diphenyl disulfide and 0.6 mmol (0.0684 g) of trifluoroacetic acid were dissolved in 3 mL of acetonitrile to obtain a homogeneous solution, which was added to a syringe; syringe set up On the syringe pump, inject the mixed solution in the syringe at a flow rate of 0.3mL / min, and then uniformly mix it with air through the gas pressure regulating valve and flow into the microchannel reaction device; place the microchannel reaction device in a 30W blue light LED light irradiation; the reaction volume of the microchannel reaction device is V=3mL, and the reaction time is 10min; after the reaction in the microchannel reactor goes through one cycle, the reaction liquid is collected, and the product yield is 70% calculated by the HPLC method, and the volume ratio is 2 : 1 ethyl acetate / saturated aqueous sodium chloride solution (V=10mL×3) for extraction, and after concentrat...
Embodiment 3
[0042] Synthesis of Example 3 Compound 2a:
[0043] 0.3 mmol (0.0763 g) of compound 1a, 0.3 mmol (0.0655 g) of diphenyl disulfide and 0.6 mmol (0.0900 g) of trifluoromethanesulfonic acid were dissolved in 3 mL of acetonitrile to obtain a homogeneous solution, which was added to a syringe; The syringe is placed on the syringe pump, and the mixed solution in the syringe is injected at a flow rate of 0.3mL / min, and then uniformly mixed with air through the gas pressure regulating valve and then flows into the microchannel reaction device; the microchannel reaction device is placed in 30W The reaction volume of the microchannel reaction device was V=3mL, and the reaction time was 10min; after the reaction in the microchannel reactor went through a cycle, the reaction liquid was collected, and the product yield was calculated by HPLC as 56%, and the volume was 56%. Extracted with 2:1 ethyl acetate / saturated aqueous sodium chloride solution (V=10 mL×3), concentrated and separated by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com