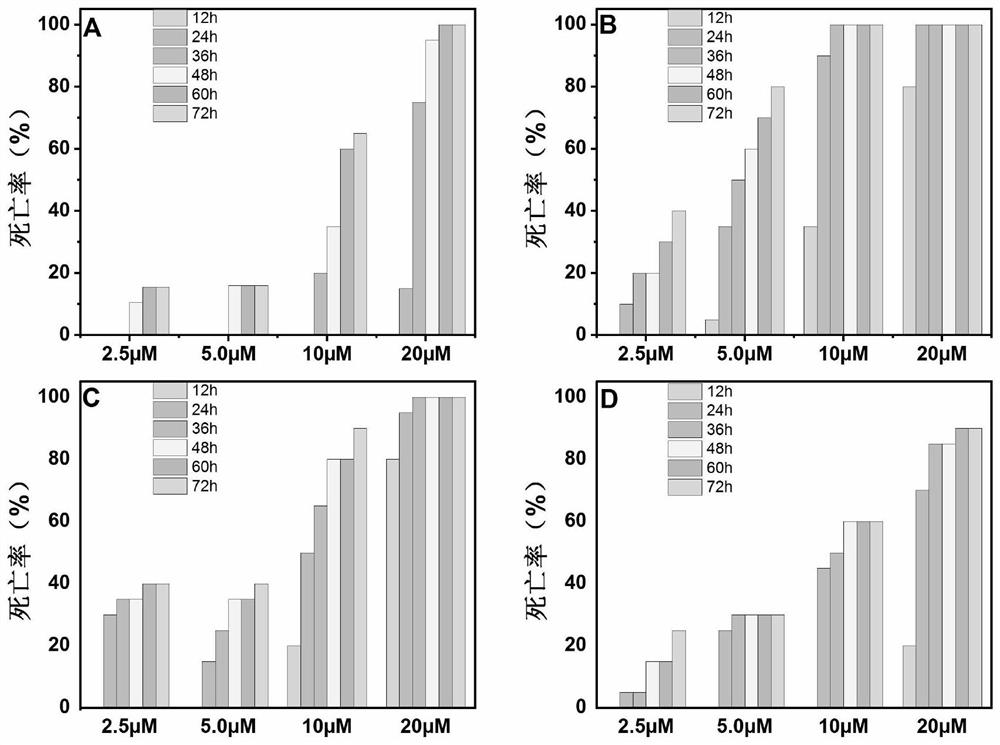
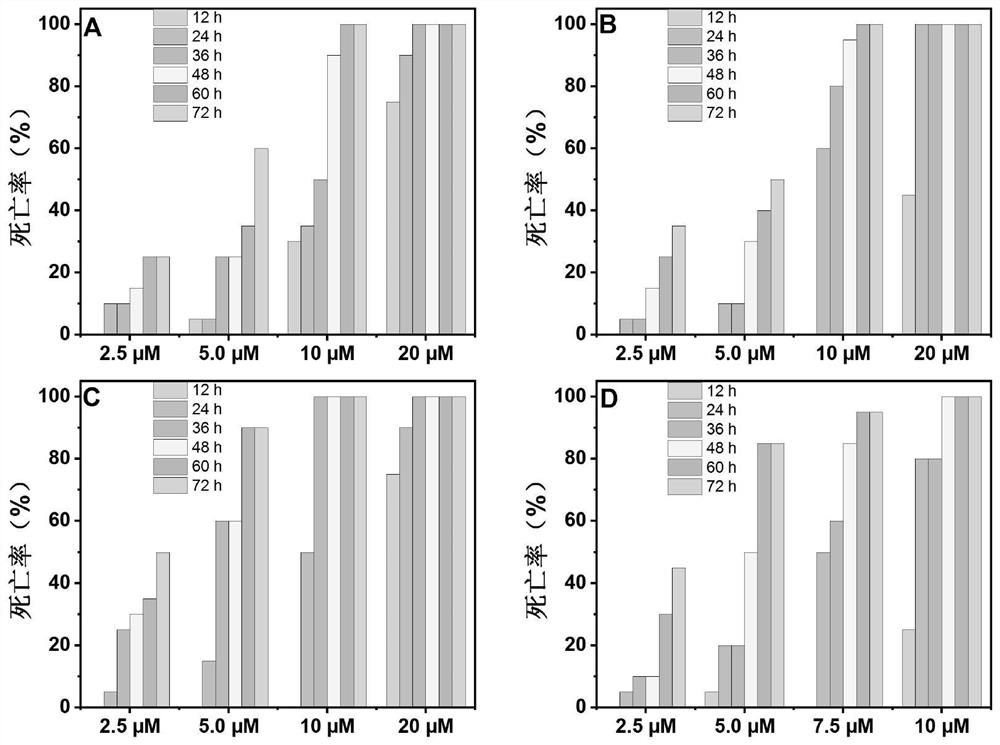
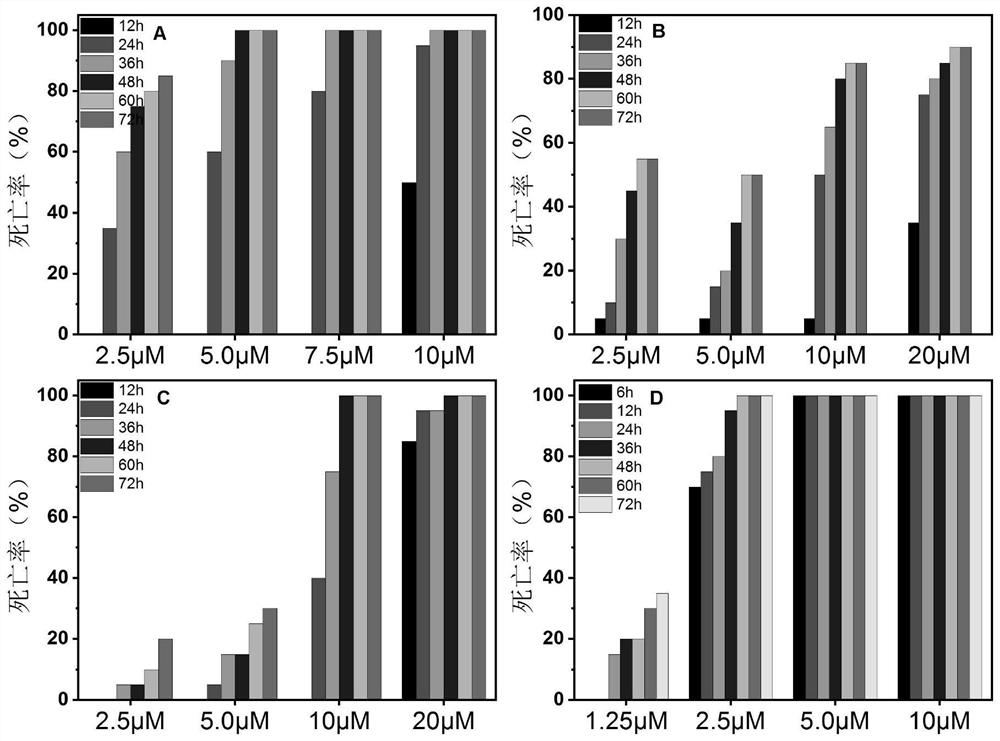
Application of piperlongumine compound in preparation of medicine for treating schistosomiasis japonicas and medicine composition
A technology of longamine base and schistosomiasis, which is applied in the field of drug treatment, can solve the problems of the advent of clinical drugs and the lag in the research and development of new drugs for schistosomiasis japonica.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] In the present invention, the preparation method of the piperamine base compound preferably comprises the following steps:
[0039] When X is H, the substituted phenylacrylic acid, oxalyl chloride and the first solvent are mixed, and an acyl chloride reaction is carried out to obtain a phenylacryloyl chloride compound;
[0040] Mixing the phenylacryloyl chloride compound with the lactam lithium salt solution, and carrying out a first nucleophilic substitution reaction to obtain the longamine base compound;
[0041] When X is not H, mix substituted phenylacrylic acid, pivaloyl chloride, triethylamine and the second solvent, and carry out acidolysis reaction to obtain an acid anhydride compound;
[0042] Mixing the acid anhydride compound with the lactam lithium salt solution, and carrying out a second nucleophilic substitution reaction to obtain a longamine base compound;
[0043] The substituted phenylacrylic acid has the structure shown in formula 1; the lactam in the...
Embodiment 1
[0112] Synthesis of Longinaminine (Compound 1):
[0113] In a 50mL round-bottomed flask, add 20mL of anhydrous dichloromethane and 0.95g (4mmol) of 3,4,5-trimethoxyphenylacrylic acid (the second substituted phenylacrylic acid), dropwise add 5mL (59mmol) of oxalyl chloride, 30 After 5 hours of reaction at ℃, vacuum distillation was performed to obtain 3,4,5-trimethoxybenzene acryloyl chloride;
[0114] Under nitrogen protection, 0.19g (2mmol) of 5,6-dihydropyridin-2(1H)-one (the first lactam) was dissolved in 10mL of anhydrous tetrahydrofuran, cooled to -78°C; then 1.3mL was slowly added (2.2mmol) lithium diisopropylamide, react at -78°C for 45min to obtain a lithium lactam salt solution; add 4mmol of 3,4,5-trimethoxyphenylacryloyl chloride, continue to react at -78°C for 1h, then warm to room temperature The reaction was carried out for 12 h; after the reaction, 10 mL of saturated ammonium chloride solution was added to quench the reaction, extracted with dichloromethane, dri...
Embodiment 2
[0117] Synthesis of Compound 2
[0118] 10 mL of anhydrous dichloromethane and 0.95 g (4 mmol) of 3,4,5-trimethoxyphenylacrylic acid (the second substituted phenylacrylic acid) were added to a 25 mL round-bottomed flask, and 2.5 mL (29.5 mmol) was added dropwise with stirring. Oxalyl chloride, refluxed at 40°C for 4 hours, and then distilled under reduced pressure to obtain 3,4,5-trimethoxyphenylacryloyl chloride;
[0119] Under nitrogen protection, 0.26g (2mmol) of 3-chloro-5,6-dihydro-2(1H)-one (the first lactam) was dissolved in 5mL of anhydrous tetrahydrofuran, cooled to -78°C; then slowly Add 2.6 mL (4.4 mmol) of lithium diisopropylamide and react for 45 min to obtain a lithium lactam salt solution, add 4 mmol of 3,4,5-trimethoxybenzene acryloyl chloride, continue to react at -78°C for 2 hours, then warm to room temperature and react for 16 hours After the reaction, 10 mL of saturated ammonium chloride solution was added to quench the reaction, extracted with dichlorom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com