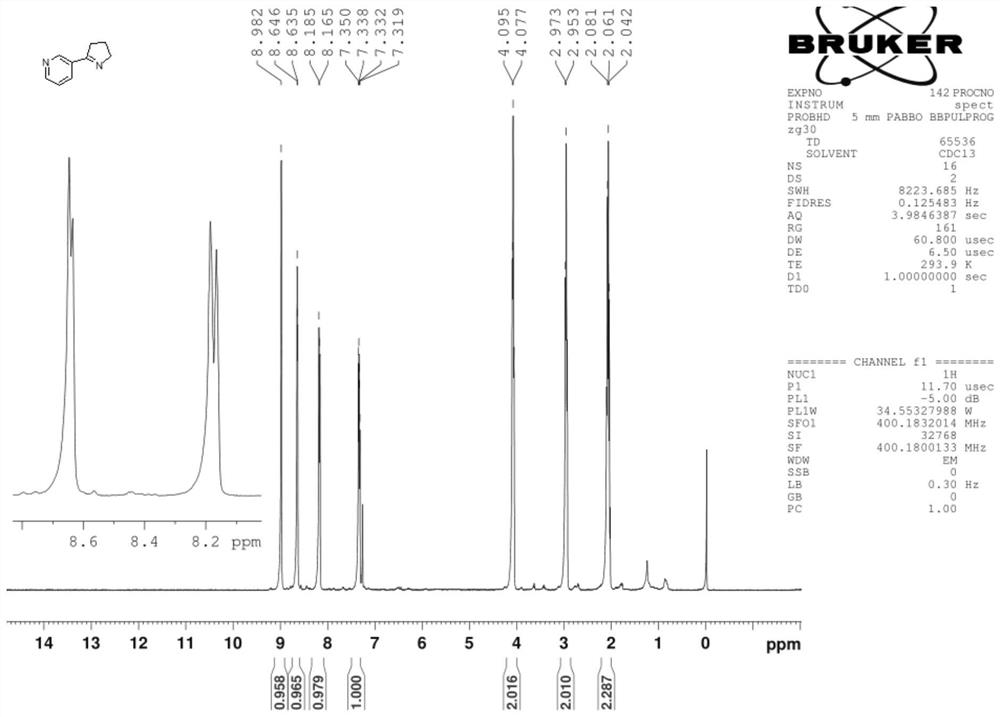
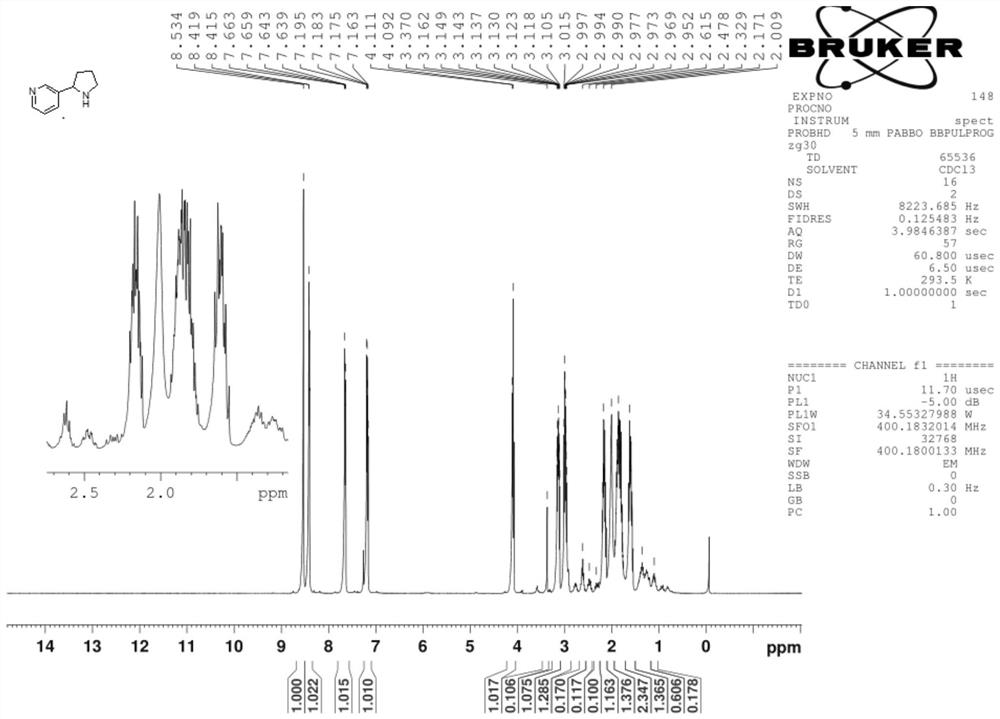
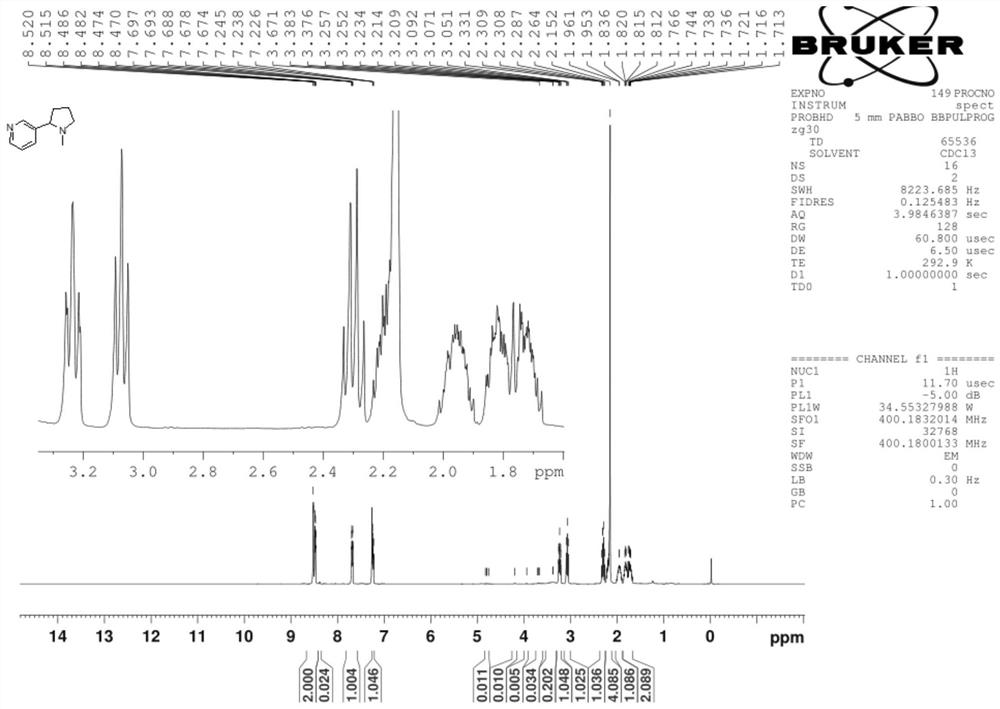
Synthesis process of nicotine with single configuration
A synthesis process and configuration technology, applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, organic chemistry, etc., can solve the problem of high synthesis cost, achieve short reaction time and high e.e value , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0037] The preparation method of mesmin is described below by taking the preparation example 1 of mesmin as an example.
[0038] Preparation example 1 of mesmin provides a kind of preparation method of mesmin, and its synthetic route is as follows:
[0039] The specific preparation steps are:
[0040] Under nitrogen protection, 24.6 g of nicotinic acid was added to the reactor, and then 24.1 g of thionyl chloride was added dropwise for 1-2 hours. After the dropwise addition was completed, the temperature of the reaction system was increased to 70 °C, and the reaction was kept for 2 hours. , the excess thionyl chloride was removed under reduced pressure, and then the temperature was lowered to 25 ° C to obtain 28.2 g of nicotinyl chloride;
[0041] Add 150 g of trimethylbenzene, 18.5 g of pyrrolidone and 22.4 g of triethylamine to the four-necked flask, and then add 28.2 g of nicotinyl chloride dropwise. The reaction was carried out for 6h, then the temperature was lowered to...
Embodiment 1
[0050] The specific preparation steps are:
[0051] Under nitrogen protection, CuF (PPh) was added to the reactor 3 ) 32.0g of solid 2MeOH, 1g of L11 ligand, 250g of tetrahydrofuran, stirred at 25°C for 0.5h, added 20ml of PMHS liquid, and stirred at 25°C for 0.5h until the system turned dark red, then cooled the system to -10°C, Subsequently, 50 g of 50wt% maxmin tetrahydrofuran solution (maxmin and tetrahydrofuran solution 1:1) was added dropwise, the dropping time was 0.5h, and the dripping was completed at -10°C and stirred until the system turned yellow. At this time, maxmin reacted completely. ;
[0052] After the reaction of maxmin was completed, 1.5mol / mol of NH was added 4 F solution 200ml, stir for 1.0h to hydrolyze excess PMHS, and the hydrolysis reaction generates silyl ether;
[0053] The copper salt in the solution was removed by filtration under reduced pressure with 15 g silica gel, and the filter cake was washed with dichloromethane (2×10 mL DCM). The filt...
Embodiment 2
[0058] The specific preparation steps are:
[0059] Under nitrogen protection, CuF (PPh) was added to the reactor 3 ) 3 2.0g of 2MeOH solid, 1g of L12 ligand, and 250g of toluene, stirred at 25°C for 0.5h at a rotating speed, added 20ml of PMHS liquid, and stirred at 25°C for 0.5h until the system turned dark red, then cooled the system to 0°C, Subsequently, 50g of 50wt% maxesmin tetrahydrofuran solution (1:1 of maxmin and tetrahydrofuran solution) was added dropwise, and the dropping time was 0.5h.
[0060] After the reaction of maxmin was completed, 1.5mol / mol of NH was added 4 F solution 200ml, stir for 1.0h to hydrolyze excess PMHS, and the hydrolysis reaction generates silyl ether;
[0061] The copper salt in the solution was removed by filtration under reduced pressure with 15 g silica gel, and the filter cake was washed with dichloromethane (2×10 mL DCM). The filtrate was separated into phases, and the aqueous phase was extracted with dichloromethane (3×100 mL), and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com