Ammonium-modified silicon oxide loaded boron oxide for oxidative dehydrogenation of propane and preparation method of ammonium-modified silicon oxide loaded boron oxide
A technology for oxidative dehydrogenation and silicon oxide carrier, applied in chemical instruments and methods, physical/chemical process catalysts, organic chemistry, etc. It is convenient for large-scale production and application, good stability, and the effect of increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0038] The boron oxide / ammonium modified MCM-41 catalyst described in this example (theoretical mass ratio B 2 O 3 :MCM-41=1:10) preparation process is as follows:
[0039] (1) Weigh out ammonium pentaborate ((NH 4 ) 2 B 10 O 16 ·8H 2 O) 0.16 g, dissolved in 11 mL of 2.5 mol / L ammonia solution to obtain a boron-containing solution.
[0040] (2) To the solution obtained in (1), add 1.0 g of the above-mentioned MCM-41 synthesized with ammonia water as an alkali source, rapidly stir in a 35°C water bath for 3 hours, centrifuge, and dry the solid in an oven at 80°C to obtain a catalyst precursor, and then add It was calcined in a muffle furnace at 550 °C for 2 h to obtain a boron oxide / ammonium modified MCM-41 catalyst.
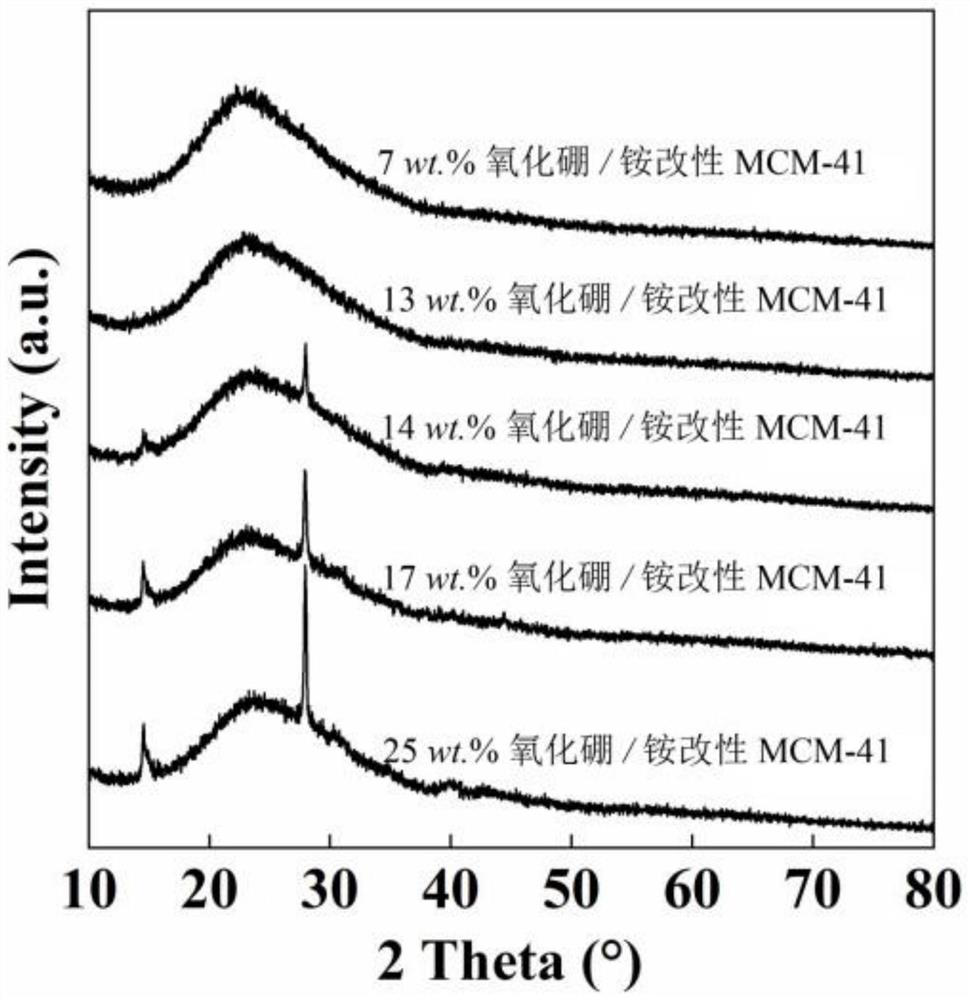
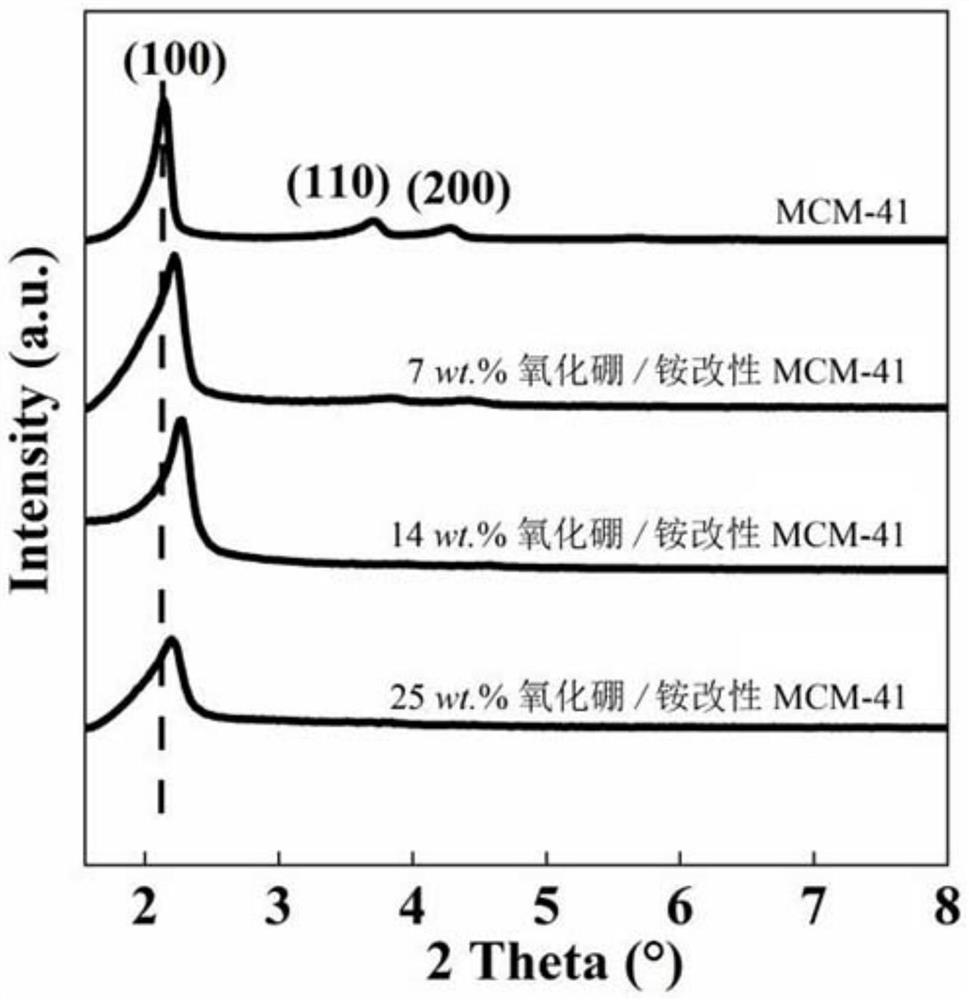
[0041] B of the boron oxide / ammonium modified MCM-41 catalyst prepared in this example 2 O 3 The actual content is 7wt.%, the specific surface area is 732m 2 / g, pore volume 0.70cm 3 / g, the wide-angle XRD pattern is as follows figure 1 As shown, the ...
Embodiment 1-2
[0043] The boron oxide / ammonium modified MCM-41 catalyst described in this example (theoretical mass ratio B 2 O 3 :MCM-41=2:10) preparation process is as follows:
[0044] (1) Weigh out ammonium pentaborate ((NH 4 ) 2 B 10 O 16 ·8H 2 O) 0.31 g, dissolved in 11 mL of 2.5 mol / L ammonia solution to obtain a boron-containing solution.
[0045] (2) To the solution obtained in (1), add 1.0 g of the above-mentioned MCM-41 synthesized with ammonia water as an alkali source, rapidly stir in a 35°C water bath for 3 hours, centrifuge, and dry the solid in an oven at 80°C to obtain a catalyst precursor, and then add It was calcined in a muffle furnace at 550 °C for 2 h to obtain a boron oxide / ammonium modified MCM-41 catalyst.
[0046] B of the boron oxide / ammonium modified MCM-41 catalyst prepared in this example 2 O 3 The actual content is 13wt.%, and the specific surface area is 565m 2 / g, pore volume 0.60cm 3 / g, the wide-angle XRD pattern is as follows figure 1 shown.
Embodiment 1-3
[0048] The boron oxide / ammonium modified MCM-41 catalyst described in this example (theoretical mass ratio B 2 O 3 :MCM-41=3:10) preparation process is as follows:
[0049] (1) Weigh out ammonium pentaborate ((NH 4 ) 2 B 10 O 16 ·8H 2 O) 0.47 g, dissolved in 11 mL of 2.5 mol / L ammonia solution to obtain a boron-containing solution.
[0050] (2) To the solution obtained in (1), add 1.0 g of the above-mentioned MCM-41 synthesized with ammonia water as an alkali source, rapidly stir in a 35°C water bath for 3 hours, centrifuge, and dry the solid in an oven at 80°C to obtain a catalyst precursor, and then add It was calcined in a muffle furnace at 550 °C for 2 h to obtain a boron oxide / ammonium modified MCM-41 catalyst.
[0051] B of the boron oxide / ammonium modified MCM-41 catalyst prepared in this example 2 O 3 The actual content is 14wt.%, the specific surface area is 550m 2 / g, pore volume 0.54cm 3 / g, the wide-angle XRD pattern is as follows figure 1 As shown, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com