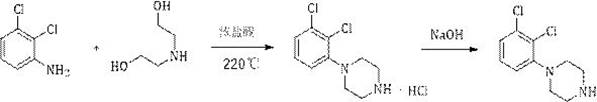
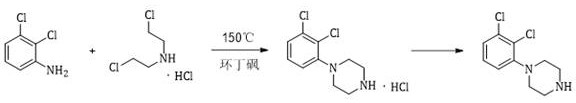
Preparation method of 1-(2, 3-dichlorophenyl) piperazine
A technology of dichlorophenyl and dichloroaniline, which is applied in the field of preparation of 1-piperazine, can solve the problems of relatively large harm to the human body and the environment, high equipment requirements, serious problems, etc., so as to avoid harm to people and the environment, and the product The effect of high purity and yield and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of Compound 1
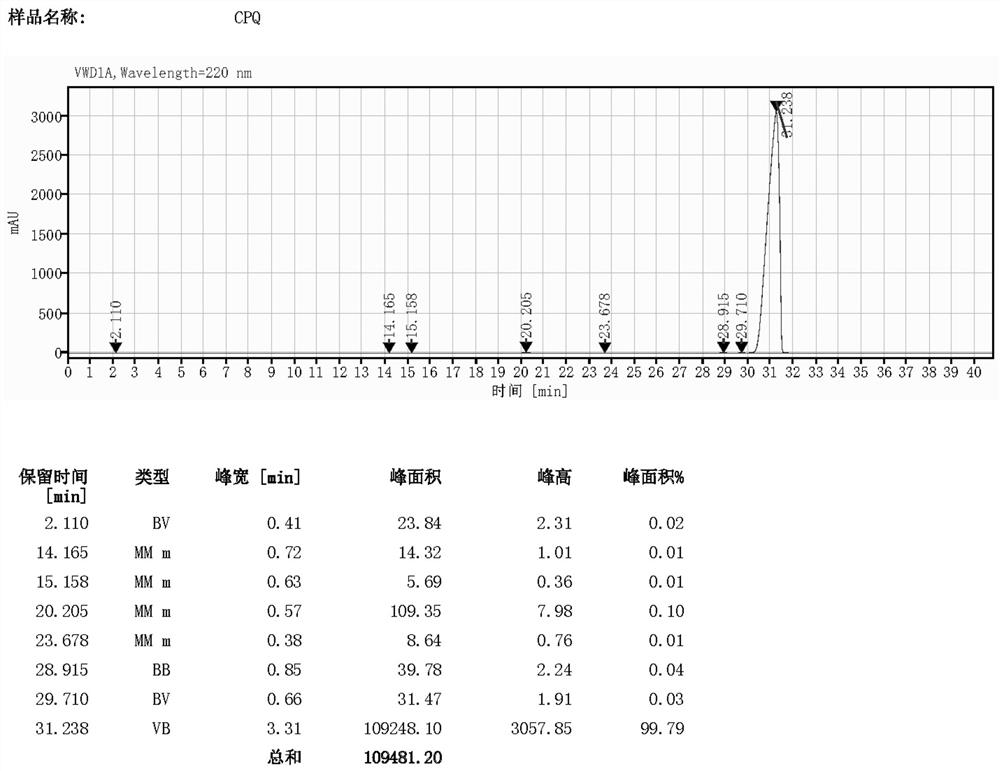
[0043] In a 500mL reaction flask, add 105.5g of diethanolamine, heat it to 120-130°C, and pass 240g of hydrogen bromide gas. At 150°C, 135.5 g of 2,3-dichloroaniline was slowly added dropwise for about 6 hours. After the dropwise addition, continue to react until the molar amount is “2 / (2+1) is less than 5%”; after the reaction ends, slowly add 20% sodium hydroxide dropwise to pH=9-10, and keep 1 at 90-100°C 300 mL of toluene was added and stirred for 1 hour; left to stand for stratification, and the water layer was discarded; the organic layer was recovered solvent toluene, and the residue was subjected to vacuum distillation, and the fractions at 170-175 °C / 10 mmHg were collected to obtain 137.6 g of compound 1, HPLC The purity was 99.79%, and the yield was 71.2%.
[0044] The reaction temperature of diethanolamine and hydrogen bromide can be selected in the range of 100-200°C, and the hydrolysis temperature can be selected in the range of ...
Embodiment 2
[0049] Synthesis of Compound 1
[0050] In a 1000mL reaction flask, add 105.5g diethanolamine and 600g 48% hydrobromic acid solution, the reaction mixture is heated to 120-130°C for 3 hours; then the temperature is raised to 150-160°C, and 135g of 2,3 - Dichloroaniline, about 6 hours. After the dropwise addition, continue to react until the molar amount is "2 / (2+1) less than 5%"; after the reaction is over, slowly add 20% sodium hydroxide dropwise to pH=9-10, and keep 1.5 at 90-100°C 300 mL of toluene was added and stirred for 1 hour; left to stand for stratification, and the aqueous layer was discarded; the organic layer was decompressed to recover solvent toluene, and the residue was subjected to vacuum distillation to collect fractions at 170-175 °C / 10 mmHg to obtain 122.3 g of compound 1 , the HPLC purity was 99.70%, and the yield was 63.5%.
Embodiment 3
[0052] Synthesis of Compound 1
[0053]In a 1000mL reaction flask, add 105.5g diethanolamine and 135.2g 2,3-dichloroaniline, heat the reaction mixture to 120-130°C, and slowly introduce 230g hydrogen bromide gas, the exotherm is very obvious and needs to be controlled Introduce speed reaction for 3 hours; then heat up to 130-150 ° C, continue to react until the molar amount "2 / (2+1) is less than 5%"; after the reaction is over, slowly add 20% sodium hydroxide dropwise to pH=9 -12, keep at 90-100 ℃ for 1 hour; add 300 mL of toluene and stir for 1 hour; let stand for stratification, and discard the water layer; the organic layer is decompressed to recover solvent toluene, and the residue is subjected to vacuum distillation to collect 170-175 ℃ / 10 mmHg fraction, 126.5 g of compound 1 was obtained, the HPLC purity was 99.56%, and the yield was 65.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com