Method for detecting related substances of 3-amino-6-methoxypyridazine
A methoxypyridazine and detection method technology, which is applied in the field of medicine, can solve the problems of no relevant literature reports on the detection method of process impurities, etc., and achieve the effects of good linear correlation, strong specificity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 System suitability test
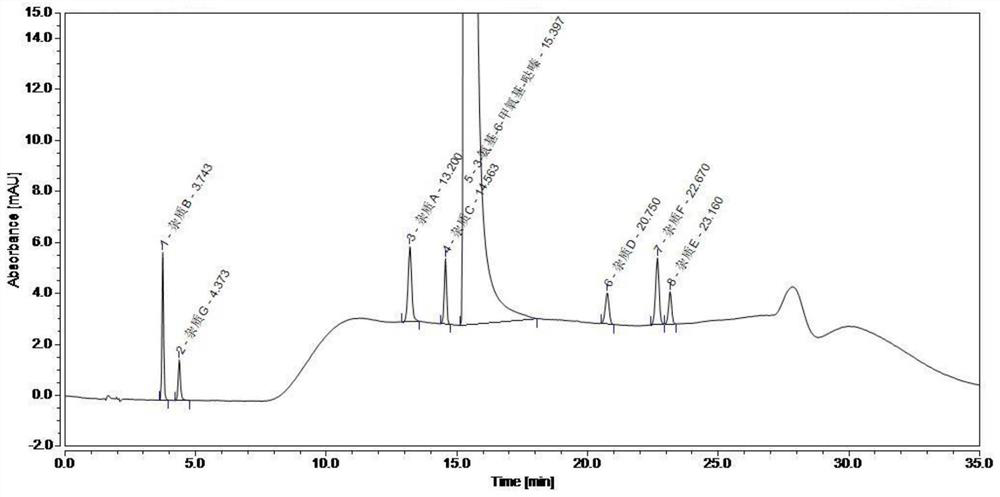
[0046] Preparation of system suitability solution: take about 25 mg of 3-amino-6-methoxypyridazine test product, accurately weigh it, put it in a 50 ml volumetric flask, add water and ultrasonic to dissolve, cool to room temperature, and then add 7.5 μg / 5ml of each impurity stock solution of ml, diluted with water to the mark, shaken up to prepare a system suitability solution of each impurity 0.75μg / ml and 3-amino-6-methoxypyridazine 0.5mg / ml. Sample injection test, detection conditions are as follows: chromatographic column: Atlantis T3 column; mobile phase: use 20mmol / L potassium hexafluorophosphate buffer (adjust pH to 3.0 with phosphoric acid) as mobile phase A, use acetonitrile as mobile phase B, gradient wash flow rate: 1.0ml / min; column temperature: 35°C; detection wavelength: 215nm; injection volume 5μl. Record the results as in Table 1 and figure 1 shown.
[0047] Table 1 System suitability test results
[0048] ...
Embodiment 2
[0050] Example 2 Specificity test
[0051] 2.1 Interference test
[0052] Blank solvent: water
[0053] Preparation of the test solution: Precisely weigh about 25 mg of the 3-amino-6-methoxypyridazine test product, put it in a 50ml measuring bottle, add water to ultrasonically make the solution, cool to room temperature, then dilute with water to the mark, shake well .
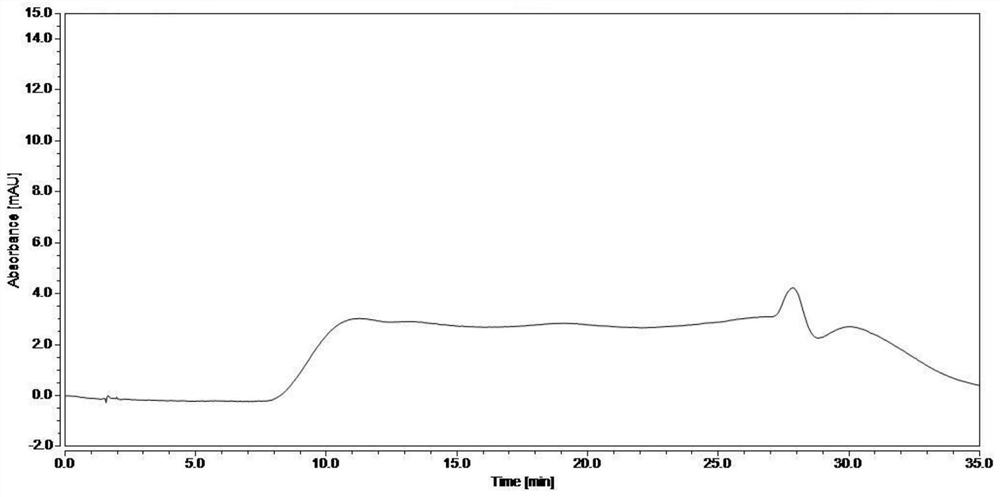
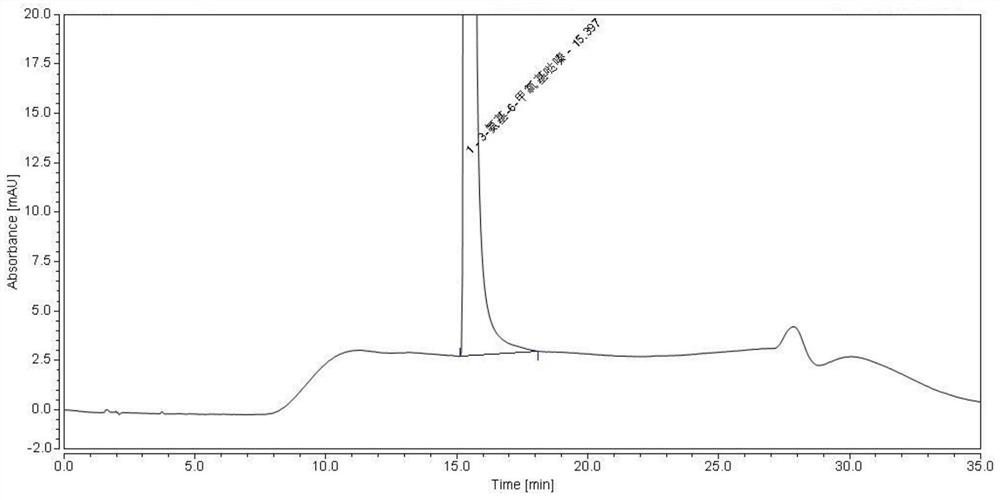
[0054]The blank solvent and the test solution were injected separately, and the detection conditions were as follows: chromatographic column: Atlantis T3 column; mobile phase: 20mmol / L potassium hexafluorophosphate buffer (adjusted to pH 3.0 with phosphoric acid) as mobile phase A, with Acetonitrile is mobile phase B, gradient elution; flow rate: 1.0 ml / min; column temperature: 35°C; detection wavelength: 215 nm; injection volume 5 μl. Record the results, the spectra are as follows figure 2 and image 3 shown. The results showed that the blank solvent did not interfere with the detection of the test pro...
Embodiment 3
[0075] Example 3 Detection limit and quantification limit
[0076] Preparation of 3-amino-6-methoxypyridazine reference substance stock solution: Precisely weigh about 25 mg of 3-amino-6-methoxypyridazine reference substance, put it in a 50ml volumetric flask, add water to sonicate to dissolve, and cool to room temperature , and then dilute with water to the mark, shake well.
[0077] Preparation of quantitative limit stock solution: Precisely measure 1.5ml of 3-amino-6-methoxypyridazine reference stock solution and 5ml of other impurity stock solutions in the same 100ml measuring bottle, add water to dilute to the mark, and shake well.
[0078] Preparation of the limit of quantification solution: Precisely measure 1ml of the limit of quantification stock solution, put it in a 50ml volumetric flask, add water to dilute to the mark, and shake well.
[0079] Preparation of detection limit solution: Precisely measure 3ml of quantitation limit solution and put it in a 10ml volume...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com