Method for preparing 2, 4-dichlorobenzoic acid from propiconazole 4-H isomer
A technology of dichlorobenzoic acid and propiconazole, which is applied in the field of synthesis of 2,4-dichlorobenzoic acid, can solve the problems of hazardous waste treatment, no disposal method, etc., achieves wide range of uses, reduces production costs, and reduces hazardous wastes Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of [2-chloro-4-(4-chlorophenoxy)-phenyl]-2-[1,2,4]triazole-4-ethanone
[0038]100 g (0.29 mol) of propiconazole 4-H isomer and 112 g (0.58 mol) of 19% hydrochloric acid were put into the reaction flask, the temperature was raised to 95° C., and the reaction was incubated for 18 hours.
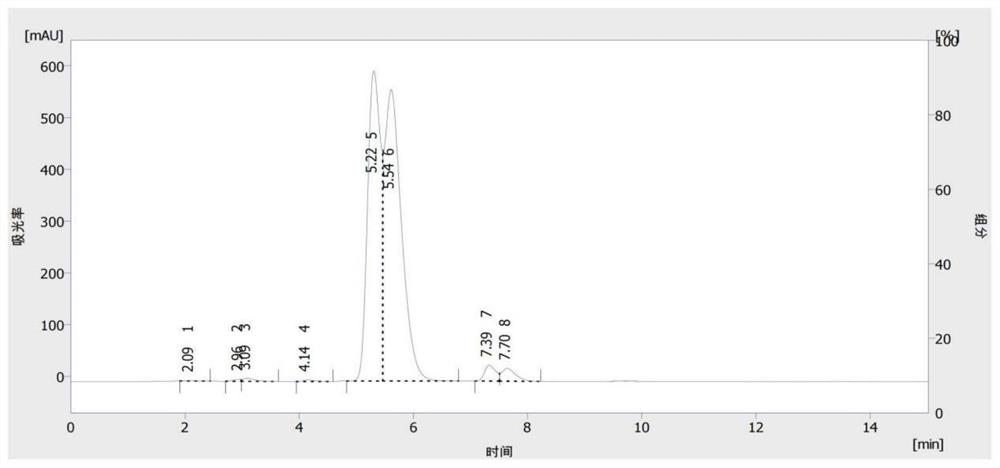
[0039] In order to confirm the reaction product of this step, after the reaction is completed, the temperature is lowered to 25-30 ° C, the reaction solution is slowly added to a 15% aqueous sodium hydroxide solution, stirred for 1 hour, the pH is measured to be 11, filtered, and the obtained solid is dried , 72.1 grams of product were obtained, the content was 96.3% by liquid analysis, and the yield was 92.8%. The product was detected by liquid chromatography, and it was proved that the product was 1-(2,4-dichlorophenyl)-2-(4H- 1,2,4-Triazol-4-yl)ethanone, such as figure 2 shown.
Embodiment 2
[0040] Example 2: Synthesis of [2-chloro-4-(4-chlorophenoxy)-phenyl]-2-[1,2,4]triazole-4-ethanone
[0041] Same as Example 1, the difference is that 19% hydrochloric acid is replaced by 72% sulfuric acid, the temperature is raised to 140°C, and other conditions and process operations remain unchanged.
[0042] As a result, 70.1 g of 1-(2,4-dichlorophenyl)-2-(4H-1,2,4-triazol-4-yl)ethanone was obtained. Liquid phase analysis and detection confirmed that the product content was 96.8%, and the calculated yield was 90.7%.
Embodiment 3
[0043] Example 3: Synthesis of [2-chloro-4-(4-chlorophenoxy)-phenyl]-2-[1,2,4]triazole-4-ethanone
[0044] Same as Example 1, the difference is that 19% hydrochloric acid is replaced by 50% methanesulfonic acid (the molar weight is unchanged), and other conditions and process operations are unchanged.
[0045] As a result, 71.3 g of 1-(2,4-dichlorophenyl)-2-(4H-1,2,4-triazol-4-yl)ethanone was obtained. Liquid phase analysis and detection confirmed that the product content was 96.5%, and the calculated yield was 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com