Bisamide derivative intermediate and preparation method of bisamide derivative
A technology of derivatives and bisamides, applied in the field of compound synthesis, can solve the problems of complex preparation process, high safety risk, unfavorable large-scale production and the like, and achieve the effects of easy control of process conditions, simple process and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] In the traditional process for preparing benfluenzamide, the process is complicated, and highly toxic substances are often used, resulting in uncontrollable process and extremely high safety risk, which is not conducive to industrialized large-scale production, such as the use of dimethyl sulfate in one technology. The preparation of bisamide derivative intermediates from hazardous chemicals requires palladium to catalyze carbonylation, and the process conditions are severe, which is not conducive to large-scale scale-up experiments; in another technology, the use of hazardous triphosgene and palladium catalyzed hydrogenation to synthesize fenflubenilamide, which is safe The risk is extremely high, and the process conditions are severe, which is not conducive to large-scale scale-up experiments.
[0061] Based on this, those skilled in the present application propose to improve the above-mentioned synthetic route to obtain a synthetic route that is relatively simple and ...
Embodiment 1
[0172] (1) the synthesis of compound (2), the synthetic route is as follows:
[0173]
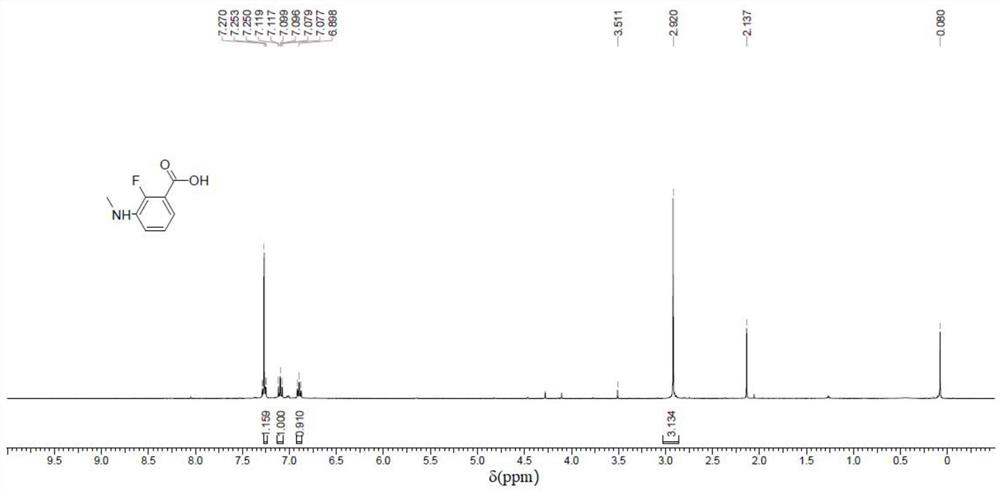
[0174] The specific steps are as follows: at 0 °C, to a mixture of 3-amino-2-fluorobenzoic acid (compound 1) (8.00 g, 51.5 mmol, 1 eq.) and concentrated sulfuric acid (50 mL) was added dropwise aqueous formaldehyde solution (9.29 g) , 37%purity, 309mmol, 8.52mL, 6eq), the internal temperature was maintained at about 30°C during the dropwise addition, after the dropwise addition, the mixture was stirred at 40°C for reaction, and LCMS was used to monitor the reaction at any time, and the reaction was 2 After one hour, solid sodium hydroxide was added to the reaction solution until pH=3, then extracted with ethyl acetate (25 mL*3), and the combined organic phases were concentrated to obtain the crude product.
[0175] The crude product was subjected to column chromatography (100-200 mesh silica gel, petroleum ether / ethyl acetate volume ratio=1:1) to obtain 2-fluoro-3-methylaminobenzoic acid...
Embodiment 2
[0216] Embodiment 2 is basically identical with embodiment 1, and difference is: step (2) is as follows:
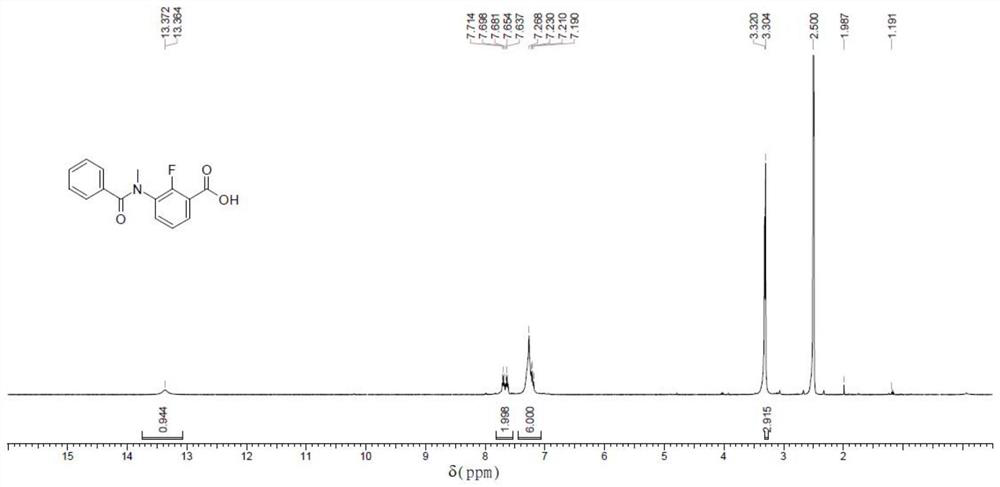
[0217] Compound 2 (100 mg, 591.18 μmol, 1 eq) was dissolved in tetrahydrofuran (1.0 mL) to prepare a solution, then pyridine (93.52 mg, 1.18 mmol, 95.43 μL, 2 eq) and compound 3 (benzoyl chloride, 99.72 mg, 709.42 μmol were added) , 82.41 μL, 1.2eq), and then the reaction was stirred at 60 °C for 12 hours, and the results are shown in Table 1 below.
[0218] Table 1
[0219]
[0220] According to the analysis in Table 1, it can be seen that the substance with the retention time of 0.363 is compound 4, and the specific mass spectrum is as follows Figure 9 As shown, LC-MS for compound 4: (M+1)+: 274.0.
[0221] Combined with the above detection results, it can be known that the yield of compound 4 in the above steps is about 10%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com