NO donor type piperlongumine derivative as well as preparation method and application thereof
A technology of perylene amide and derivatives, applied in the field of medicinal chemistry, can solve the problems of short biological half-life and limit direct application, and achieve good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
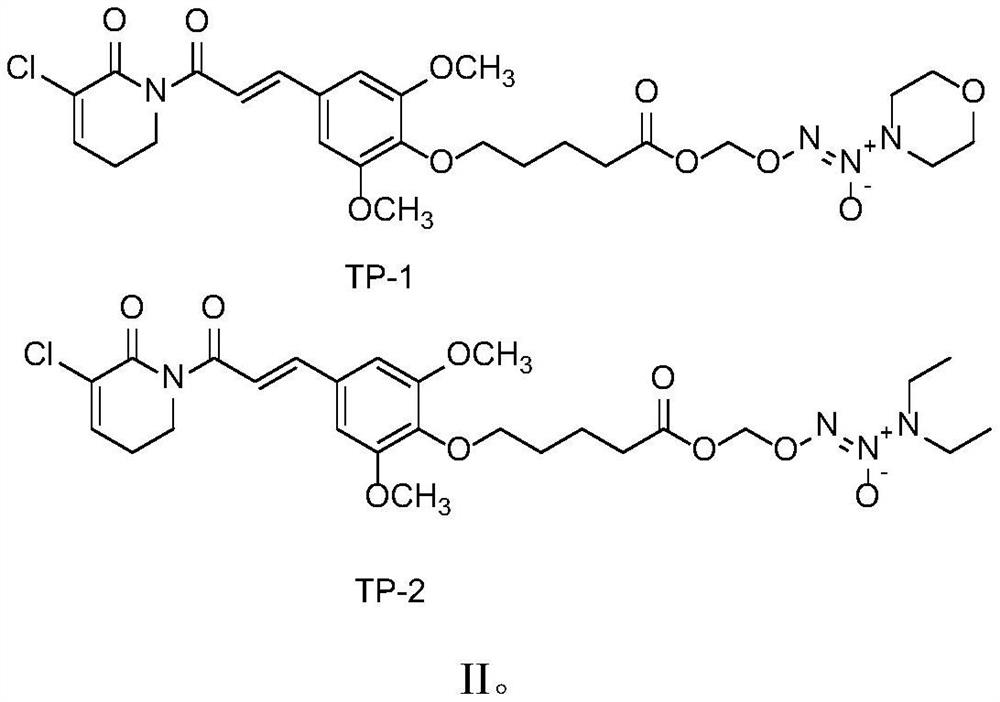
[0037] In this example, two compounds were synthesized, which were abbreviated as TP-1 and TP-2 respectively.
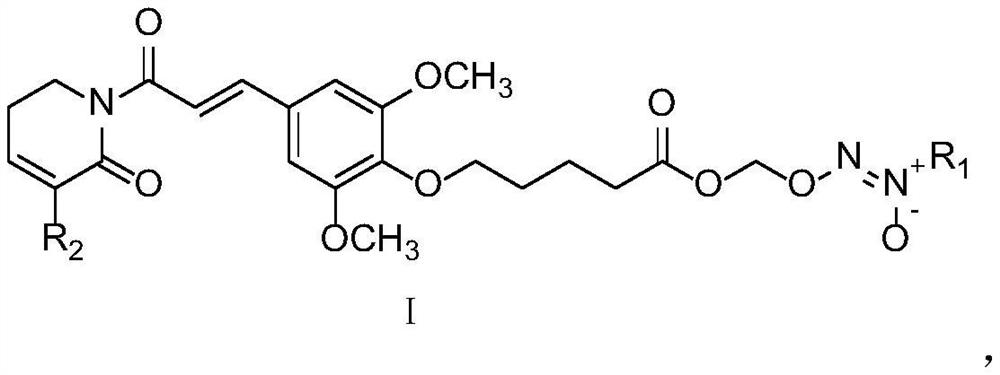
[0038] The name of compound TP-1 is: 2-((5-(4-(3-(5-chloro-6-oxo-3,6-dihydropyridin-1(2H)-yl)-3-oxopropane -1-en-1-yl)-2,6-dimethoxyphenoxy)pentane(oxy)methoxy)-1-morpholinodiazene 1-oxide, the structural formula is as follows:
[0039]
[0040] The name of compound TP-2 is: 1-((5-(4-(3-(5-chloro-6-oxo-3,6-dihydropyridin-1(2H)-yl)-3-oxopropane -1-En-1-yl)-2,6-dimethoxyphenoxy)pentane(oxy)methoxy)-3,3-diethyltriazole-1-ene 2-oxide , the structure is as follows:
[0041]
[0042] The reaction scheme involved in the present embodiment of the present invention is as follows:
[0043]
[0044] Step a: Add anhydrous sodium sulfate (2.35 g, 16.54 mmol) to the flask, add 15 ml of dichloromethane, mix well, add concentrated sulfuric acid (1.63 g, 16.63 mmol), and stir at room temperature for 1 h. A mixture of 5-bromovaleric acid (1.50 g, 8.29 mmol) and tert-butan...
Embodiment 2
[0061] Example 2: Control compound (3-chloro-1-(3-(4-((5-((diethylamino)oxy)-5-oxopentyl)oxy)-3,5-di Synthesis of Methoxyphenyl)acryloyl)-5,6-dihydropyridin-2(1H)-one))(TP-3)
[0062] Compound structure:
[0063]
[0064] Take 0.2 g of intermediate 8 into a round-bottomed flask, add dichloromethane to dissolve, add 0.041 g of N,N-diethylhydroxylamine (DEHA), after mixing, add 0.094 g of EDC under an ice bath, and stir at room temperature The reaction was carried out for 8 h, and the reaction was monitored by TLC. After extraction, the organic layer was collected and washed with water and saturated sodium chloride (20 ml x 3), respectively, dried over anhydrous sodium sulfate, and concentrated. Purification by thin layer chromatography, developing solution ethyl acetate: petroleum ether = 2: 1, to obtain 18 mg of the product as a yellow paste. 1 H NMR (600MHz, CDCl 3 )δ7.72–7.65 (dd, J=15.5, 2.1Hz, 1H), 7.43–7.36 (dd, J=15.5, 2.1Hz, 1H), 7.09–7.05 (td, J=4.6, 2.0Hz, 1H) ...
Embodiment 3
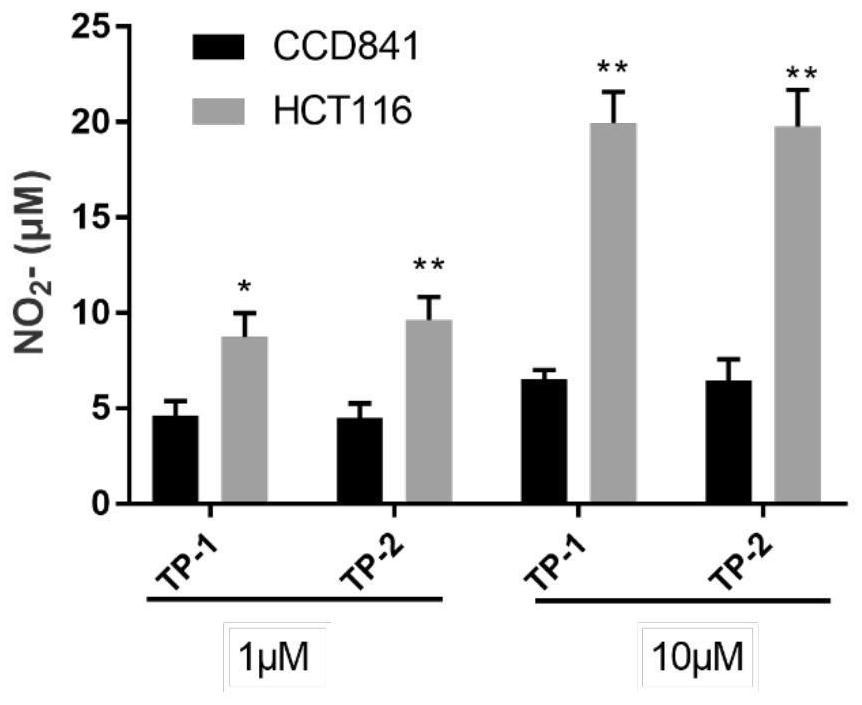
[0065] Example 3: Partial pharmacological tests and results of representative compounds of the present invention
[0066] 1. In vitro anti-tumor screening
[0067] Cell viability was determined by MTT assay. The cells in the logarithmic growth phase were adjusted to a density of 5000 cells / well, seeded in a 96-well plate, placed at 37°C, 5% CO 2 Cultivated under conditions until the cells were 90% confluent, then incubated with serum-free medium (HCT-116:McCoy's 5A medium, A549:F12K medium, CCD841:EMEM medium) for 2h to synchronize the cells, using the corresponding compounds respectively. After treating the cells for 24h and 72h, carefully remove the supernatant, add 90μL of fresh culture medium, and then add 10μL of MTT solution, 37°C, 5% CO 2 Incubate for 4 h under conditions, after the incubation, aspirate the supernatant, add 110 μL of Formazan lysis solution to each well, and place on a shaker for low-speed shaking for 10 min to fully dissolve the crystals. The absorb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com