Phenyl polydiacetylene acid as well as preparation method, application and recovery thereof
A technology of phenyl polydiynoic acid and diynoic acid, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of unfavorable phenyl polydiynes The commercial production and practical application of similar materials can improve product performance, improve the photocatalytic degradation effect, and realize the effect of recycling and regeneration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation of PBDA
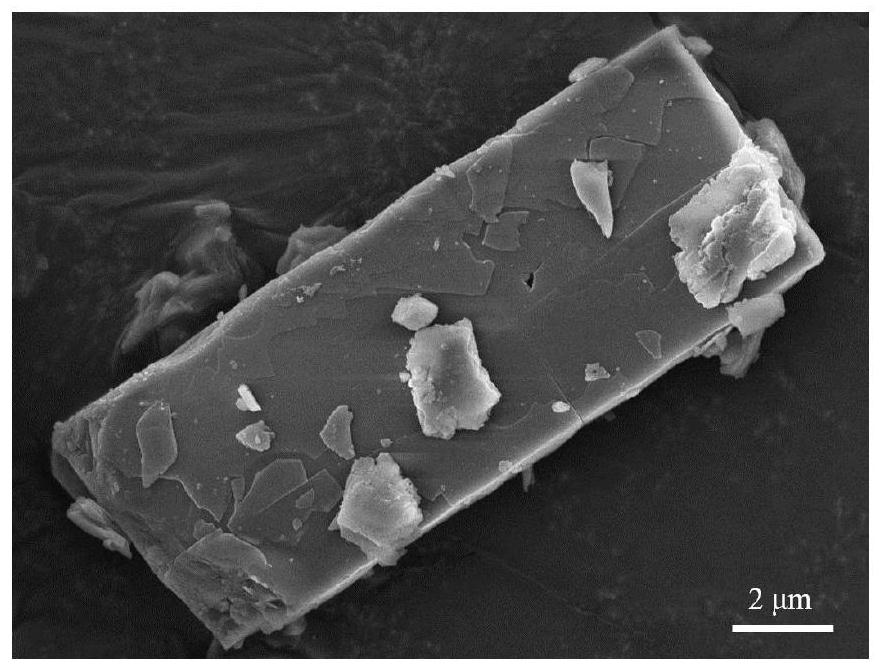
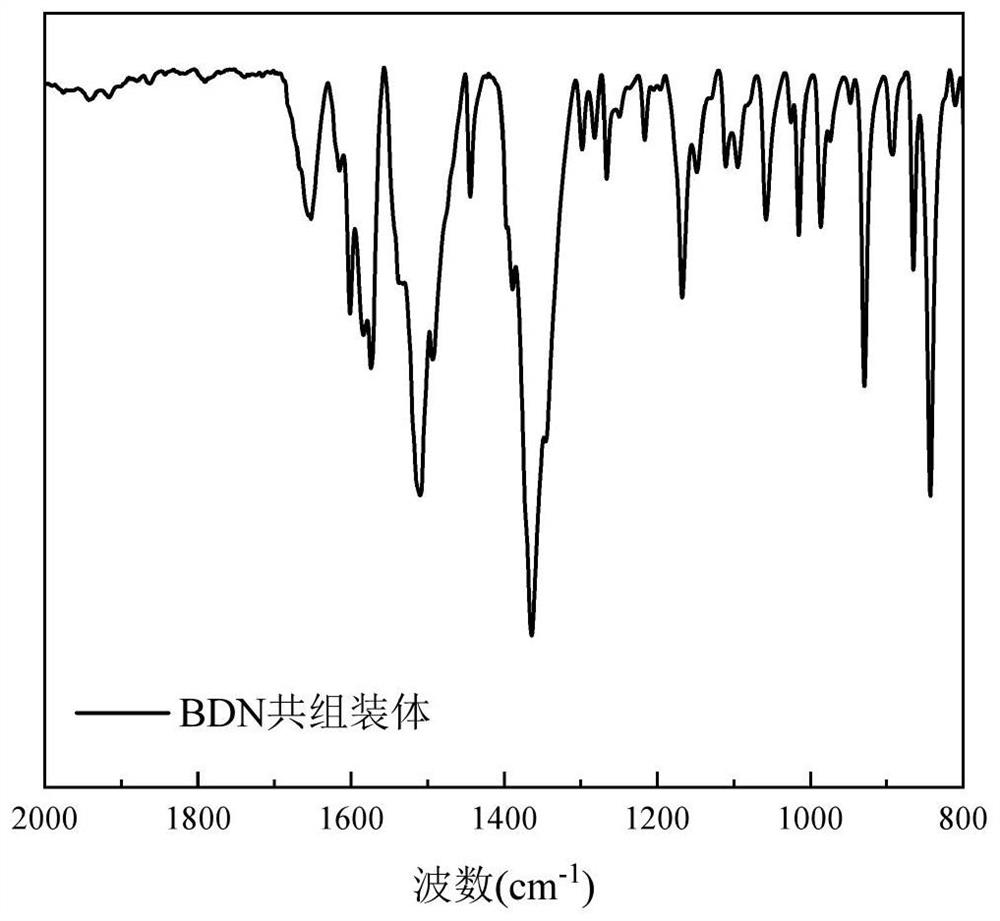
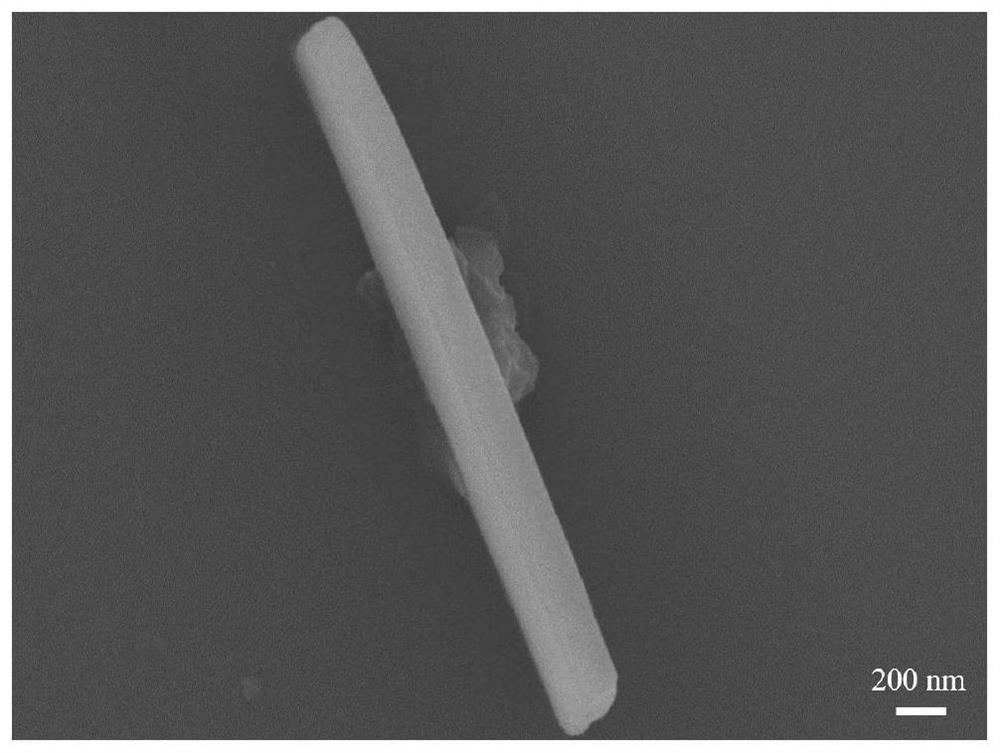
[0044] 30 mg of BDA monomer powder was weighed into a 10 mL glass bottle, and 6 mL of DMF solvent was added to prepare a 5 mg / mL BDA solution. The BDA solution was heated in a 90°C water bath for 10 min, taken out after the solution turned into a white emulsion, and cooled to 25°C at room temperature. To the white emulsion was added 151.55 μL of NMA monomer. Quickly shake and mix evenly, and the solution turns into a yellow transparent liquid. After standing at 25°C for 24h, yellow particles were precipitated in the solution. The solution was placed under a 365nm ultraviolet mercury lamp for 1 hour of irradiation. After the irradiation, 100 μL of 12 mol / L hydrochloric acid was added to the solution. Shake and mix evenly, centrifuge and wash with deionized water to obtain PBDA powder.
Embodiment 2
[0045] Example 2: Preparation of PBDA (BDA low concentration)
[0046] Weigh 10 mg of BDA monomer powder into a 10 mL glass bottle, add 5 mL of DMF solvent to prepare a 2 mg / mL BDA solution. The BDA solution was placed in a 90°C water bath and heated for 10 min. After the solution became clear and transparent, it was taken out and cooled to 25°C at room temperature. To this was quickly added 50.48 μL of NMA monomer. Quickly shake and mix evenly, and the solution turns into a yellow transparent liquid. After standing at 25°C for 24h, yellow particles were precipitated in the solution. The solution was placed under a 365nm ultraviolet mercury lamp for irradiation for 1 h, and after the irradiation was completed, 50 μL of 12 mol / L hydrochloric acid was added thereto. Shake and mix evenly, centrifuge and wash with deionized water to obtain PBDA powder.
Embodiment 3
[0047] Example 3: Preparation of PBDA (BDA high concentration)
[0048] 27 mg of BDA monomer powder was weighed into a 10 mL glass bottle, and 3 mL of DMF solvent was added to prepare a 9 mg / mL BDA solution. The BDA solution was heated in a 90°C water bath for 10 min, taken out after the solution turned into a white emulsion, and cooled to 25°C at room temperature. To the white emulsion was added 151.55 μL of NMA. Quickly shake and mix evenly, and the solution turns into a yellow transparent liquid. After standing at 25°C for 24h, yellow particles were precipitated in the solution. The solution was placed under a 365nm ultraviolet mercury lamp for 1 hour of irradiation. After the irradiation, 100 μL of 12 mol / L hydrochloric acid was added to the solution. Shake and mix evenly, centrifuge and wash with deionized water to obtain PBDA powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com