Levofloxacin tablet
A technology of levofloxacin tablets and levofloxacin, which is applied in the direction of antibacterial drugs, organic active ingredients, drug delivery, etc., can solve the problem of slow release effect decline, and achieve the effect of overcoming incomplete and unstable release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] Preparation of levofloxacin sustained-release tablets
[0040] After passing the raw material drug and the auxiliary materials except magnesium stearate through a 100-mesh sieve, weigh the required amount of ingredients and mix them thoroughly; add 55% ethanol to make a soft material, dry the wet granules and sieve them through a 20-mesh sieve for granulation; Add magnesium stearate and mix well; tablet (specification 500mg, hardness 9Kg).
[0041] Coating (JGB-150 coating pan, Jian brand) parameters: tablet bed temperature 40 degrees Celsius, air outlet temperature 47 degrees Celsius, atomization pressure 0.5Mpa, rotation speed 10rpm, spray volume 110g / min, coating weight gain 10%.
Embodiment 1
[0042] Example 1 Preliminary study on levofloxacin sustained-release tablets
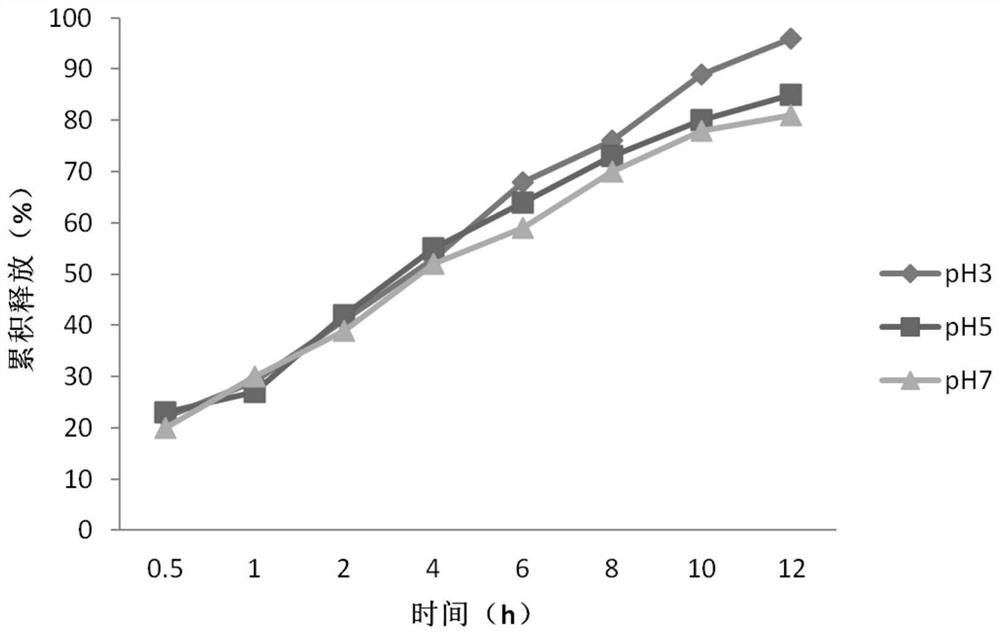
[0043] After trying a large number of sustained-release tablet formulations, the applicant constructed the following sustained-release tablet formulations, which can release the drug smoothly in about 12 hours and have good sustained-release performance (see figure 1 Curve pH3): by weight: levofloxacin hydrochloride 55%, hypromellose K100M 20%, ethylcellulose N50 15%, lactose 7.5%, magnesium stearate 2.5%.
[0044] However, further research found that the release performance of the sustained-release tablet formulation was obviously limited at higher pH, the drug could not be fully released, the release degree within 12 hours was only slightly higher than 80%, and the release amount after 8 hours was obviously insufficient (see figure 1 The curve in the pH5, pH7), which will significantly affect the efficacy and limit the convenience of drug use for patients with insufficient gastric acid or those wh...
Embodiment 2
[0045] Example 2 Study on the coating of levofloxacin sustained-release tablets
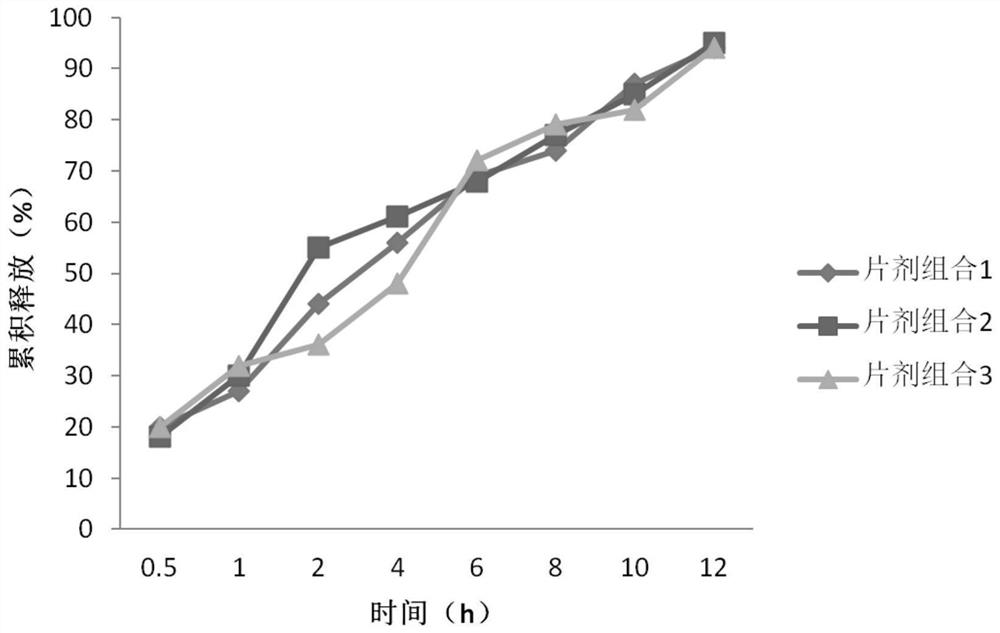
[0046] The applicant tried to solve the problem of the reference document 1 by adjusting the formula, but the effect was not satisfactory (the formulas of hypromellose K100M, ethyl cellulose N50, and lactose have been obtained by the applicant after a large number of formula screening). Applicants then attempted to use coatings to solve the above problems while providing taste-masking or taste-correcting functions to ofloxacin with a strong bitter taste. Based on production experience, the applicant has successively tried various existing coatings, such as Opadry II 85G (HPMC, aqueous solution) with 4% by weight of the tablet core, and Aquacoat ECD 30 (EC, aqueous solution) with 6% by weight of the tablet core. Except for a certain time delay, these coatings had no significant effect on the release properties of ER tablets.
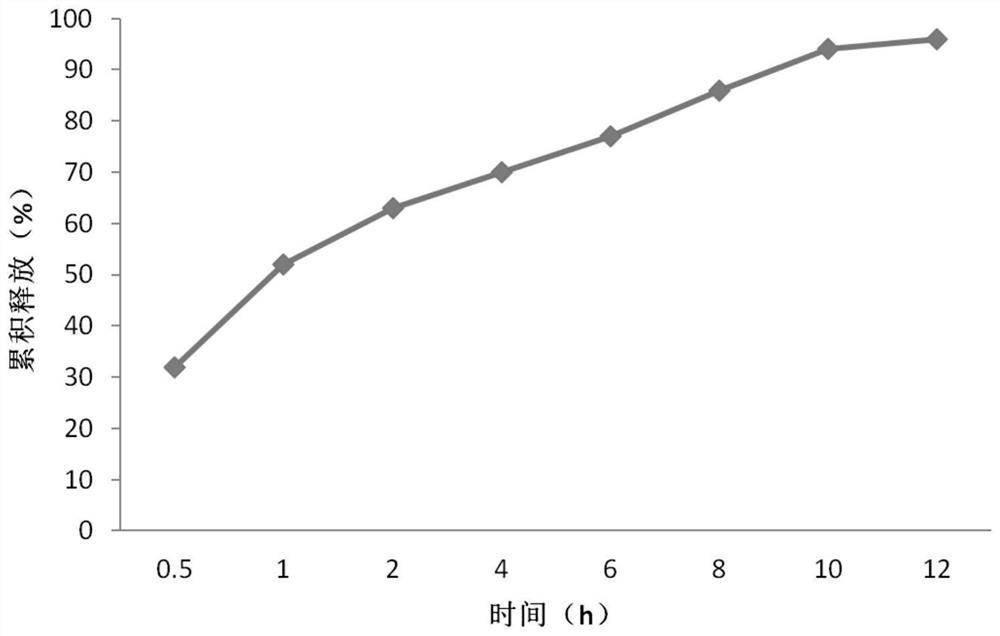
[0047]According to the characteristics of the previously used self-mad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com