C1 esterase inhibitor and preparation method thereof
An inhibitor and esterase technology, applied in the field of C1 esterase inhibitor and its preparation, can solve the problems of complex production operation, many chromatographic steps, insufficient safety, etc., so as to increase output value, save plasma resources, and improve market competition. force effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: preparation technique is as follows:
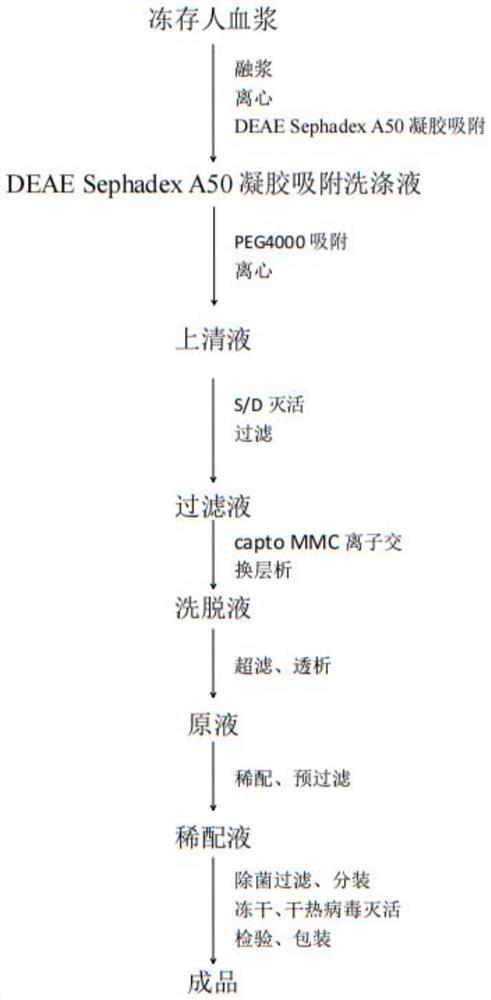
[0057] (1) After receiving 3000L of qualified human plasma during the quarantine period, the bag will be broken under the protection of laminar flow in a D-level environment. Merge it into the melting tank with circulating water at 30~35℃ for interlayer circulation melting, and control the plasma temperature between 0~4℃. After the plasma is melted, centrifuge with a centrifuge. The centrifugal force should be 6000g~20000g. The temperature is 0℃~4℃, and the effluent flow rate should be ≤4kg / min / unit, and the cryoprecipitated plasma is collected; the cryoprecipitated plasma is transferred to the adsorption tank, and the plasma temperature is controlled at 10~20℃. Plasma pH was adjusted to 7.0 ± 0.2 using acetic acid-sodium acetate buffer (pH 4.0). 45kg of equilibrated DEAESephadex A50 gel was added and adsorbed for 30 minutes under stirring. The adsorbed gel was collected in a gel column. The gel was washed 4 times ...
Embodiment 2P
[0081] Embodiment 2Optimization of PEG precipitation conditions
[0082] 2.1 Preliminary study on PEG precipitation conditions
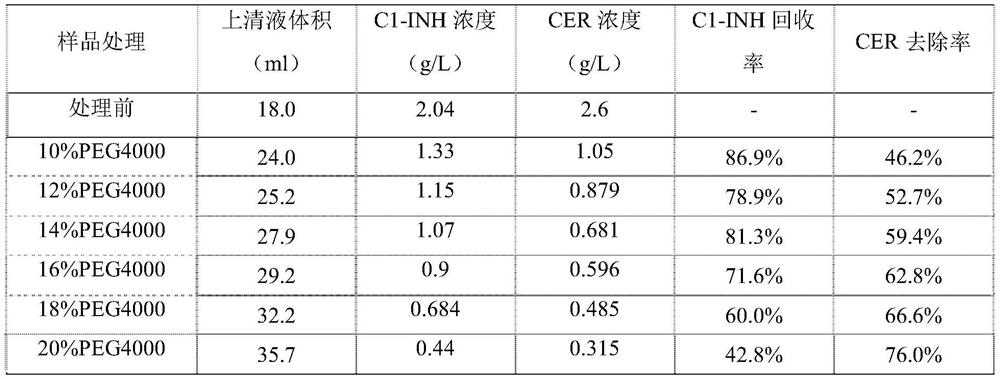
[0083] 2.1.1 After adjusting the pH of the A50 washing solution to 6.8, it was divided into 6 groups, and PEG4000 was added to the concentration of 10%, 12%, 14%, 16%, 18%, 20% (w / v), and stirred at room temperature for 30%. After 15 minutes, centrifuge at 15000g and 15°C for 20 minutes, take the supernatant for specific protein analysis, the results are shown in Table 2; another A50 washing solution is divided into 6 groups, and the pH is adjusted to 6.8, 6.6, 6.4, 6.2, 6.0, 5.5 , adding PEG4000 to a concentration of 14% (w / v), stirring at room temperature for 30 minutes, centrifuging at 15000g and 15°C for 20 minutes, and taking the supernatant for specific protein analysis. The results are shown in Table 3.
[0084] Table 2 The effect of PEG4000 concentration of 10%-20% on the content of C1-INH and CER after centrifugation at pH 6.8
[0085] ...
Embodiment 3
[0095] Example 3capto MMC ion exchange chromatography
[0096] According to the composition analysis of the sample solution, the main impurity protein was ceruloplasmin. According to the investigation of the salt concentration gradient of the washing and elution buffers, the washing buffer includes 5.88g / L sodium citrate, 3.5g / L sodium chloride; the elution buffer 5.88g / L sodium citrate, 17.52 g / L sodium chloride, under these conditions, other impurity proteins can be removed, the antigen yield of C1 esterase inhibitor can reach more than 80%, and the activity yield is more than 85%.
[0097] Table 5 capto MMC ion exchange chromatography results
[0098]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com