Berberine hydrochloride sustained-release gel and preparation method thereof
A technology of berberine hydrochloride and slow-release gel, which is applied in the direction of medical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of eczema that cannot achieve direct therapeutic effect and has poor application effect. Improve patient compliance, reduce cost, and reduce itching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The berberine hydrochloride sustained-release gel described in this example, its preparation raw materials include:
[0044] The following percentages are the percentages of each component in the total weight of the gel.
[0045] component Quality Score Berberine hydrochloride 0.2% Carbomer 940 1.5% glycerin 6.67% Tween 80 2.33% peppermint 0.3% ethyl paraben 0.4% water add to 15g
[0046] The preparation method of this embodiment comprises the following steps:
[0047] (1) Weigh the prescribed amount of Carbomer 940 and mix it in a 100mL beaker, add glycerol dropwise and stir to wet, add 9mL of water to stir, leave at room temperature for 4-6h, fully swell, and set aside.
[0048] (2) Weigh berberine hydrochloride into a small beaker, add 3 mL of 0.1 mol / L hydrochloric acid and 2 mL of 95% ethanol, heat to dissolve, and put it to room temperature for later use.
[0049] (3) adding (2) drug solution into (1), sti...
Embodiment 2
[0053] The berberine hydrochloride sustained-release gel described in this example, its preparation raw materials include:
[0054] component Quality Score Berberine hydrochloride 0.2% Carbomer 940 1.5% HPMC K4M 1% glycerin 6.67% Tween 80 2.33% peppermint 0.3% ethyl paraben 0.4% water add to 15g
[0055] The preparation method of this embodiment comprises the following steps:
[0056] (1) Weigh and mix carbomer 940 and hydroxypropyl methylcellulose in the recipe amount in a 100mL beaker, add dropwise glycerin, stir and wet, add a certain amount of water, stir, leave at room temperature for 4-6h, fully swell, and set aside.
[0057] (2) Weigh berberine hydrochloride into a small beaker, add 3 mL of 0.1 mol / L hydrochloric acid and 2 mL of 95% ethanol, heat to dissolve, and put it at room temperature for later use.
[0058] (3) adding (2) drug solution into (1), stirring fully, adding Tween-80, peppermint oil, ethy...
Embodiment 3
[0062] The berberine hydrochloride sustained-release gel described in this example, its preparation raw materials include:
[0063] component Quality Score Berberine hydrochloride 0.2% Carbomer 940 1.5% HPMC K15M 1% glycerin 6.67% Tween 80 2.33% peppermint 0.3% ethyl paraben 0.4% water add to 15g
[0064] The preparation method is the same as that of Example 2.
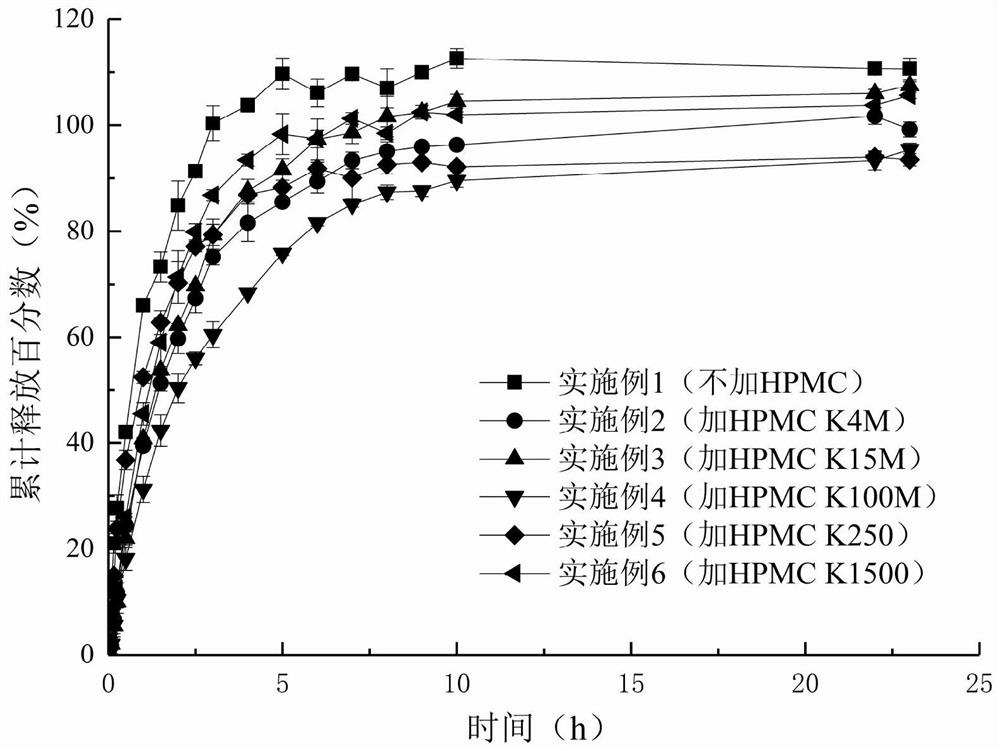
[0065] The in vitro release test method is the same as that in Example 1. In vitro release profile see figure 1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com