Synthesis method for constructing phenanthridine compound through ring opening cyclization of alkenyl benzotriazole under visible light catalysis
A technology of alkenyl benzotriazole and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of high substrate toxicity and poor substrate stability of phenanthrene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment-2-cyano-2-methyl-3-(phenidin-6-yl) ethyl propionate
[0032] Take a Schlenk reaction tube, add a magnetic stirring bar to it, and then add 0.2 mmol of 1-(1-phenylethenyl)-1H-benzo[d][1,2,3]triazole (CAS:23269- 74-1), 0.5 mmol of 4-dimethylaminopyridine (CAS: 1122-58-3), 2 mL of 1,4-dioxane (CAS: 123-91-1), and finally 0.5 mmol of Ethyl 2-bromo-2-cyanopropionate (CAS: 26526-81-8).
[0033] After argon protection, the reaction was completed under the irradiation of 450-460 nm blue LED light for 12 h. The final product was detected by TLC and finally separated by column chromatography to obtain the final product ethyl 2-cyano-2-methyl-3-(phenidin-6-yl)propanoate with a yield of 88%. The reaction equation is as follows:
[0034]
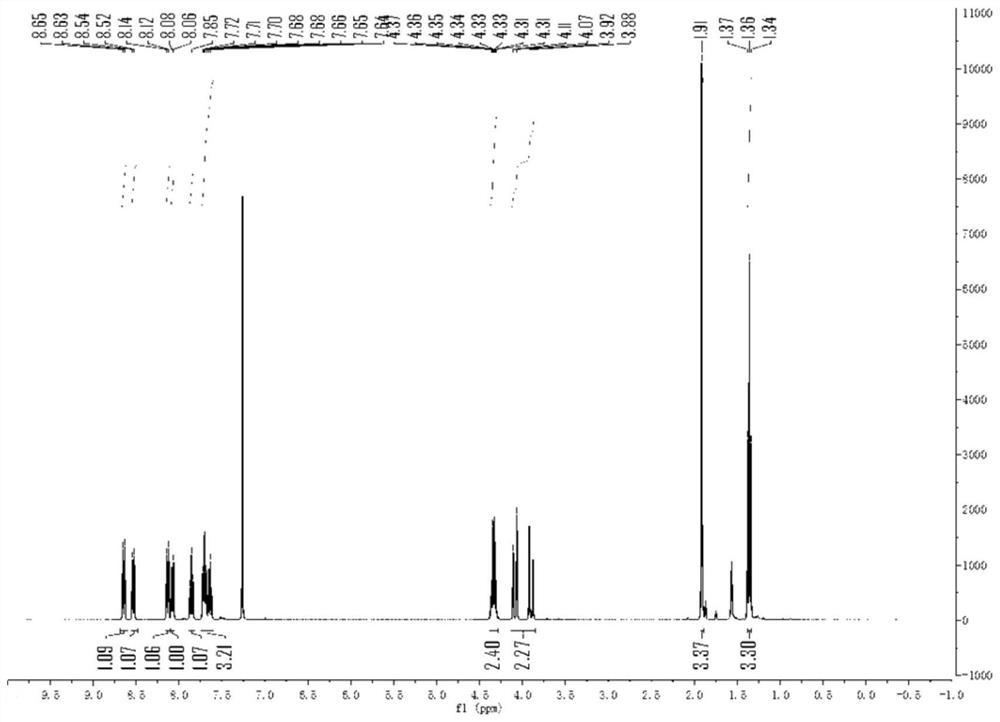
[0035] The 1H NMR characterization of ethyl 2-cyano-2-methyl-3-(phenidin-6-yl)propanoate is as follows: 1 H NMR (400MHz, CDCl 3 )δ8.64(d,J=8.3Hz,1H),8.53(d,J=7.8Hz,1H),8.13(d,J=8.2Hz,1H),8.07(d,J=7.9Hz,1H) ,7...
Embodiment 2
[0036] Example 2 Preparation of ethyl 2-cyano-2-methyl-3-(9-methylphenidin-6-yl) propionate
[0037] Take a Schlenk reaction tube, add a magnetic stirrer to it, and then add 0.2 mmol of 1-(1-(p-tolyl)ethenyl)-1H-benzo[d][1,2,3]triazole (CAS : 1186338-74-8), 0.5 mmol of 4-dimethylaminopyridine, 2 mL of 1,4-dioxane, and finally 0.5 mmol of ethyl 2-bromo-2-cyanopropionate.
[0038] After argon protection, the reaction was completed under the irradiation of 450-460 nm blue LED light for 12 h. The final product was detected by TLC and finally separated by column chromatography to obtain the final product ethyl 2-cyano-2-methyl-3-(9-methylphenidin-6-yl)propanoate with a yield of 70%. The reaction equation is as follows:
[0039]
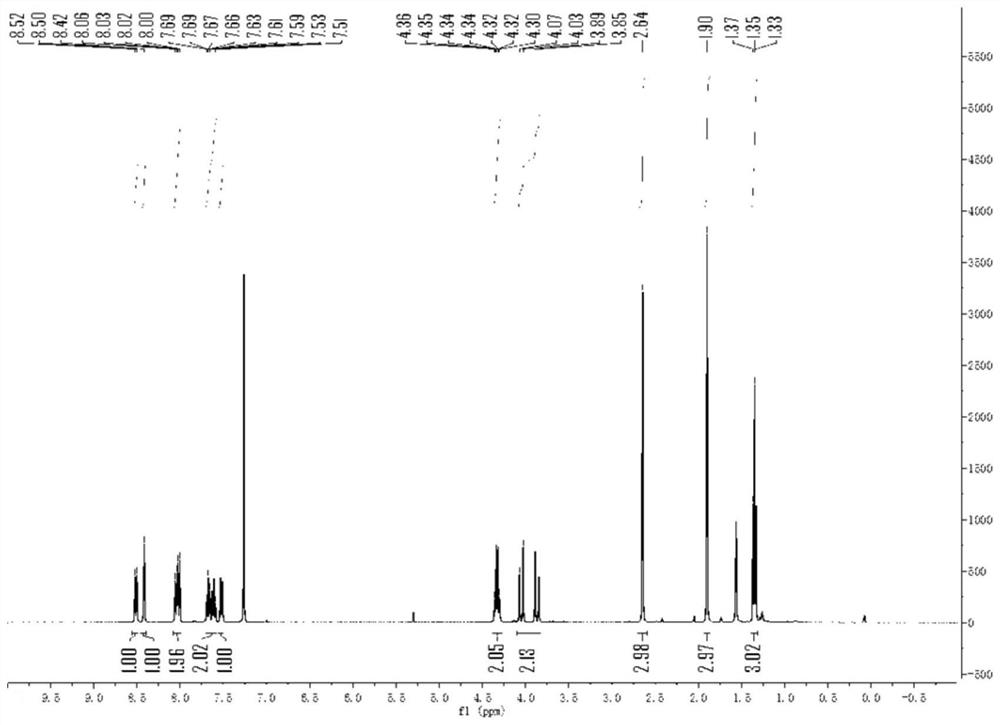
[0040] The 1H NMR spectrum of ethyl 2-cyano-2-methyl-3-(9-methylphenidin-6-yl)propanoate is characterized as follows: 1 HNMR (400 MHz, CDCl 3)δ8.51(d,J=8.0Hz,1H),8.42(s,1H),8.03(dd,J=12.4,8.3Hz,2H),7.73–7.57(m,2H),7.52(dq,J =8.3Hz,1H),4.37–4.29(m,2...
Embodiment 3
[0041] Example 3 Preparation of ethyl 2-cyano-2-methyl-3-(9-ethylphenidin-6-yl) propionate
[0042] Take a Schlenk reaction tube, add a magnetic stir bar to it, and then add 0.2 mmol of 1-(1-(p-ethylphenyl)vinyl)-1H-benzo[d][1,2,3]triazole (CAS: 1659293-79-4), 0.5 mmol of 4-dimethylaminopyridine, 2 mL of 1,4-dioxane, and finally 0.5 mmol of ethyl 2-bromo-2-cyanopropionate.
[0043] After argon protection, the reaction was completed under the irradiation of 450-460 nm blue LED light for 12 h. The final product was detected by TLC and finally separated by column chromatography to obtain the final product ethyl 2-cyano-2-methyl-3-(9-ethylphenidin-6-yl)propanoate with a yield of 70%. The reaction equation is as follows:
[0044]
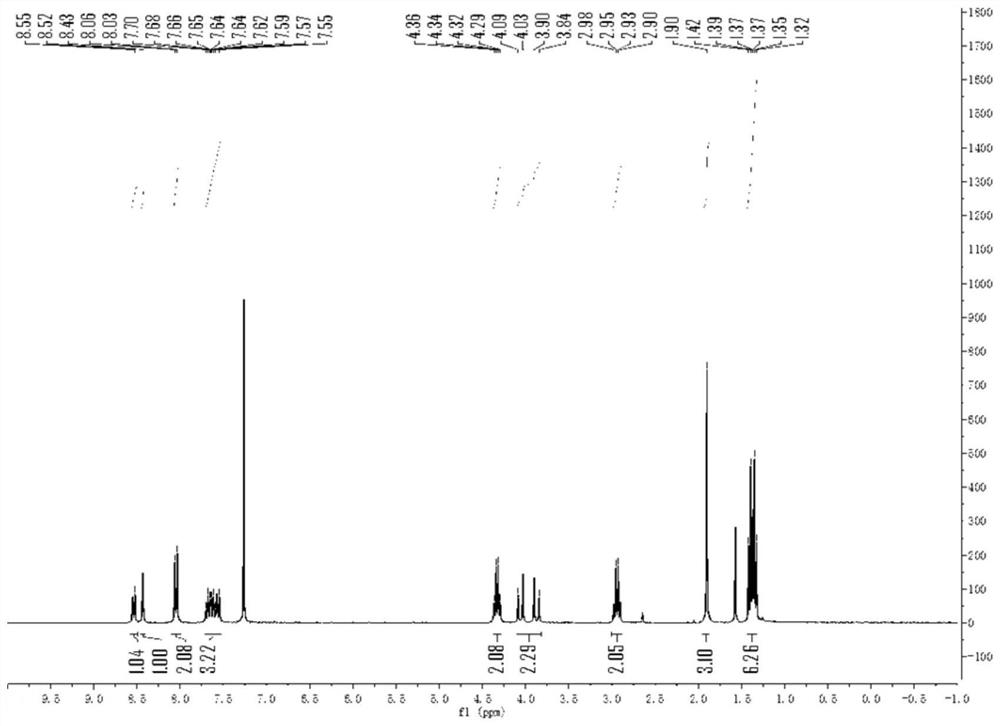
[0045] The H NMR spectrum of ethyl 2-cyano-2-methyl-3-(9-ethylphenidin-6-yl)propionate is characterized as follows: 1 HNMR (300 MHz, CDCl 3 )δ8.54(d,J=7.7Hz,1H),8.43(s,1H),8.05(d,J=8.4Hz,2H),7.73–7.51(m,3H),4.33(q,J=7.1 Hz,2H),4.03and 3.90(ABq,J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com