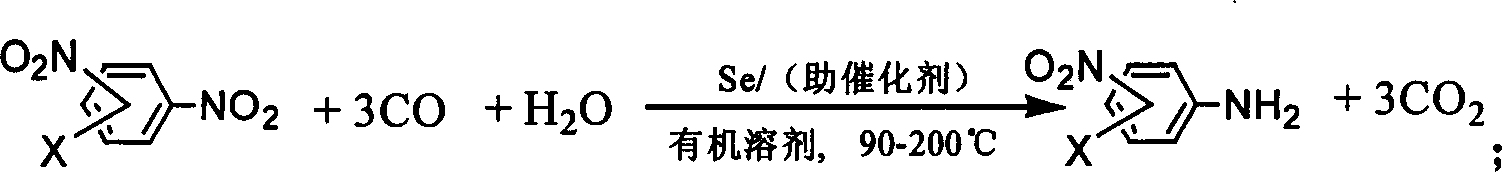
Process for synthesizing nitroarylamine compounds
A technology of nitroarylamines and compounds, which is applied in the field of synthesis of nitroarylamines to achieve the effects of high reaction selectivity, simple raw materials, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add m-dinitrobenzene (10mmol), Se (0.4mmol), H in a 100ml stainless steel autoclave 2 O (200mmol), sodium acetate (10mmol) and solvent tetrahydrofuran (15ml), after replacing 3 times with carbon monoxide, raise the pressure of carbon monoxide to 1MPa, put it into an oil bath that has risen to 160°C and stir for 3 hours, cool To room temperature, open the kettle, feed oxygen or stir in the air for 0.5-1 hour, filter out the selenium powder, concentrate the filtrate obtained by filtering, and separate with a chromatographic column to obtain m-nitroaniline. The separation yield is 83.3% ( In terms of m-dinitrobenzene), the purity is 100%.
Embodiment 2
[0026] The aromatic dinitro substance is p-dinitrobenzene, and other experimental methods and conditions are the same as in Example 1. The yield of p-nitroaniline obtained by column separation is 78.3% (calculated as p-dinitrobenzene), and the purity is 100%.
Embodiment 3
[0028] Aromatic dinitro is 2,6-dinitrotoluene, and the reaction time is 6 hours, and other experimental methods and conditions are the same as in Example 1, and the column separation to obtain 2-methyl-4-nitroaniline yield is 90.8% (with 2,6-dinitrotoluene), the purity is 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com