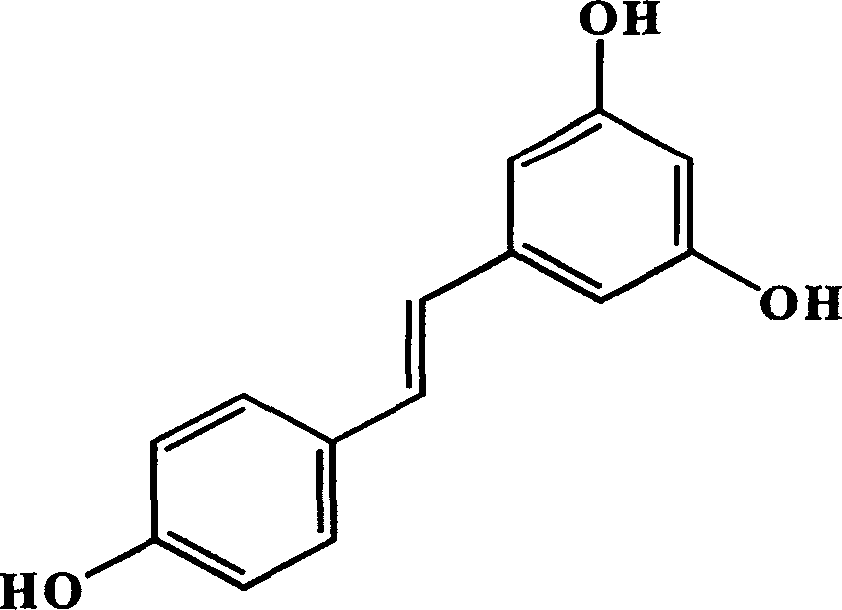
Use of trans-3,4,5-trihydroxy-diphenyl and derivant for resisting hepatitis B virus
A technology of trihydroxystilbene and hepatitis B virus, which can be applied in the directions of active ingredients of hydroxyl compounds, antiviral agents, drug combinations, etc., and can solve the problems of drug resistance, no effect, obvious toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: the extraction process of compound A
[0015] Polygonum cuspidatum 1.0kg, pulverized and leached three times with 50% methanol aqueous solution at room temperature, each time for 3 days. The filtrates were combined and filtered and then vacuum-dried to obtain 100 g of alcohol extract, which was then extracted three times with ethyl acetate (500 ml / time). The organic phases were combined and vacuum-dried to obtain 30.0 g of a paste. The paste was subjected to silica gel column chromatography with gradient elution, and the eluent was chloroform:methanol (50:1-10:1). Obtain 10 fractions, fraction 5 (3.0g) further use C 18 Column HPLC separation afforded the monomer (100 mg). Compared with the spectrum of the standard substance, it was determined to be trans-3,4',5-trihydroxystilbene.
Embodiment 2
[0016] Example 2: Compound A inhibits the secretion of HBsAg and HBeAg by L2.2.15 cells
[0017] 1 Materials and methods
[0018] 1.1 Test drugs
[0019] Drug name: trans-3,4',5-trihydroxystilbene (compound A) (molecular weight 501.48).
[0020] Drug properties: light yellow powder.
[0021] Purity: more than 95%.
[0022] Preparation method: Weigh 2mg of dry powder and add 0.2ml of DMSO to dissolve completely, then add 3.79ml of 2% DMEM to filter and sterilize.
[0023] 1.2 Cell lines and reagents
[0024] The L2.2.15 cells are preserved in this laboratory, using DMEM medium (GIBCO), supplemented with 10% fetal bovine serum, 100U / ml penicillin, 100U / ml streptomycin, G418 100μg / ml (GIBCO), 0.03% glutamine Amide, pH adjusted to 6.84 with 0.238% Hepes, Tezolan (MTT) (Sigma), Dimethylsulfoxide (DMSO).
[0025] 1.3 Drug handling
[0026] Disperse L2.2.15 cells into a single cell suspension with 0.06% trypsin, press 3×10 4 Cells / well concentration were inoculated in 96-well...
Embodiment 3
[0065] Embodiment 3: the effect of compound A anti-duck hepatitis B virus in vivo
[0066] 1 Materials and methods
[0067] 1.1 Test drugs
[0068] Compound name: trans 3,4',5-trihydroxystilbene.
[0069] Preparation: the concentration is 1.2mg / ml. Preparation method: Weigh 109mg of Compound A, add 3ml of DMSO to dissolve, and then add 67ml of water.
[0070] 1.2 Positive drugs
[0071] Drug name: Lamivudine (3TC).
[0072] Production unit: Glaxo UK.
[0073] Drug lot number: HK-42075.
[0074] 1.3 Instruments and reagents
[0075] NC membrane, purchased from Amersham Company. The DHBV plasmid was extracted by our technicians. The gap translation kit was purchased from Promega. α- 32 p-dCTP was purchased from Beijing Yahui Company. SephadexG-50, purchased from Pharmacia Company. 96-well hybridization spotter: product of Bio-Rad, USA. Geiger counter: product of S.E.International Company in the United States.
[0076] 1.4 Experimental animals
[0077] Male 1-day-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com