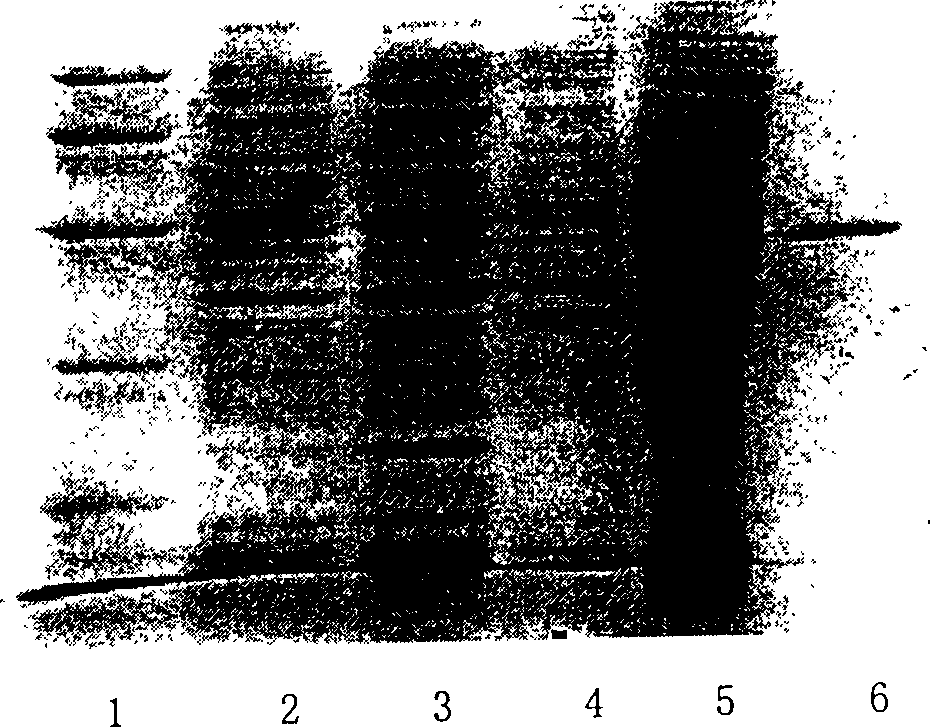Coding sequence of arisaema agglutinant protein and its application
A kind of Tiannanxing, coding technology, applied in the field of preparation of the protein and nucleic acid sequence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Cloning of ah-Lectin gene from Araceae
[0056] 1. Tissue separation (isolation)
[0057] The stems of Araceae come from Shanghai University of Traditional Chinese Medicine. After taking the inflorescence material of Aracea inflorescences, they are immediately frozen in liquid nitrogen. .
[0058] 2. RNA isolation (RNA isolation)
[0059] Take part of the tissue, grind it with a mortar, add it to a 50mL tube filled with lysate, shake it fully, and then transfer it into a glass homogenizer. After homogenization, transfer to a new 50ml tube and extract total RNA (TRIzol Reagents, Gibco, NY, USA). The quality of total RNA was identified by formaldehyde denaturing gel electrophoresis.
[0060] 3. Cloning of Full-length cDNA
[0061] According to the conserved amino acid sequences of snowdrops and other araceae lectins, degenerate primers were designed, and the principle of homologous gene cloning was used to adopt the RACE method (GibcoBRL kit)
[0062] Perform cDNA f...
Embodiment 2
[0072] Sequence information and homology analysis of ah-Lectin gene in Araceae
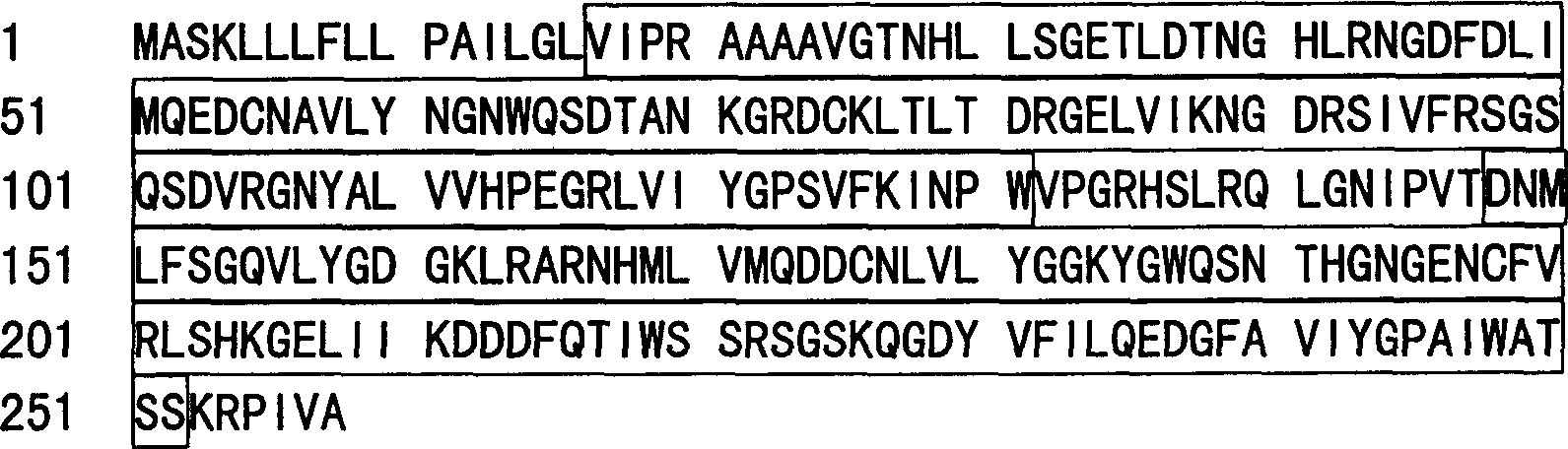
[0073] The length of the novel Aracea ah-Lectin full-length cDNA of the present invention is 1169 bp, and the detailed sequence is shown in SEQ ID NO.3, wherein the open reading frame is located at nucleotides 85-861. The amino acid sequence of Aracea ah-Lectin was deduced according to the full-length cDNA, which has a total of 258 amino acid residues, a molecular weight of 28464 Daltons, and a pI of 7.71. See SEQ ID NO.4 for the detailed sequence.
[0074] The full-length cDNA sequence of ah-Lectin and its encoded protein were identified by BLAST program in the Non-redundant GenBank+EMBL+DDBJ+PDB and Non-redundant GenBank CDS translations+PDB+SwissProt+Superdate+PIR databases. Source search found that it has a certain homology with the gene of Pinellia lectin (PTA) of Araceae (Yao et al., Acta Fudan Sinica 2001, 40: 461-464) and the gene of GNA of Amaryllidaceae sex. At the nucleotide level, i...
Embodiment 3
[0076] Study on the Structure and Function of Araceae ah-Lectin Protein
[0077] 1. Amino acid sequence domain analysis of Aracea ah-Lectin protein. The amino acid sequence of Aracea ah-Lectin protein was searched for domains in the NCBI database (web address: http: / / www.ncbi.nlm.nih.gov / Structure / cdd / wrpsb.cgi), and the following results were obtained:
[0078]
[0079] (1) In the amino acid sequence, the following domain regions are present:
[0080] Boxed region (27-131, 148-252): Lectin protein functional module (Lectin).
[0081] (2) Function analysis
[0082] There is a functional module of Lectin protein in the amino acid sequence of the gene, so it can be predicted that it does have the corresponding function.
[0083] 2. Signal peptide analysis of araceae lectin protein. Through the comparative analysis of the nucleotide sequence and amino acid sequence of the araceae lectin protein and other plant lectins, it was found that the gene of the araceae lectin prote...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com