Total synthesizing meethod for pyrrole heterocyclic alkaloid aldisin
A synthesis method and alkaloid technology, applied in the direction of organic chemistry, etc., can solve the problems of 2-pyrrolecarboxylic acid being expensive, difficult to disperse reactants, uneven dissolution, etc., and achieve low cost, short reaction time, and simple and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
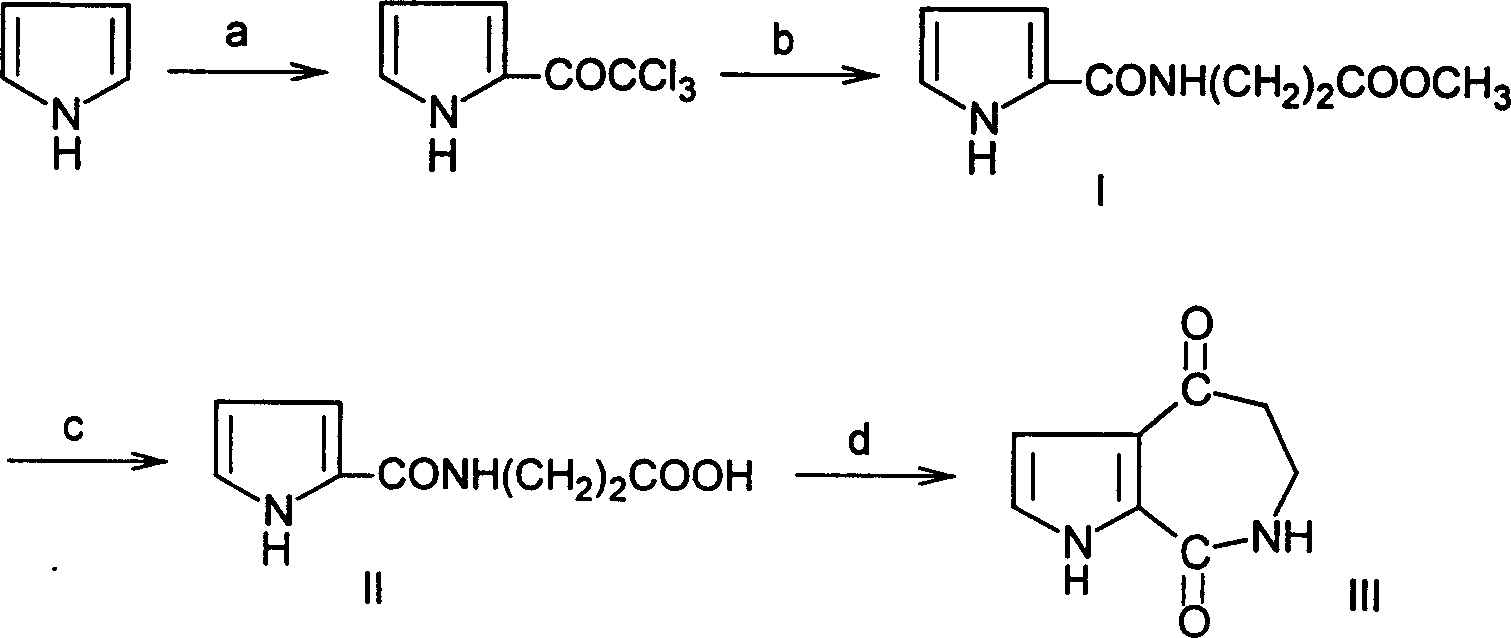
[0040] (1) Synthesis of 2-trichloroacetylpyrrole: 4.5g (55mmol) freshly steamed pyrrole, 30mL anhydrous ether, mix well, slowly drop 10g (55mmol) trichloroacetyl chloride in 20mL anhydrous ether solution under stirring, 0.5 The dropwise addition was completed within 1 hour, and stirred at room temperature for 3 hours. Stop the reaction, slowly add 4.5g K dropwise under stirring 2 CO 3 30mL aqueous solution, separate the ether layer; the aqueous layer was extracted with 15mL ether, the combined organic phases were washed with anhydrous Na 2 SO 4 Let dry overnight. Filter to remove Na 2 SO 4 . Diethyl ether was recovered from the filtrate by distillation, and the residue was recrystallized from n-hexane to obtain 9.82 g of gray flaky 2-trichloroacetylpyrrole with a yield of 84% and a melting point of 74-75°C. (yield index and physical properties are consistent with literature [Denis M.Bailey; Robert E.Johnson; and Noel F.Albertson, Org.Syn., Vol.51, 1971, 100.])
[0041]...
Embodiment 2
[0048] (1) Synthesis of 2-trichloroacetylpyrrole: same as Example 1
[0049] (2) Synthesis of N-(2-pyrroleformyl)-beta-alanine methyl ester (I)
[0050] 2.27g of β-alanine methyl ester, 4.25g of 2-trichloroacetylpyrrole and 20mL of ethyl acetate were added to a 50mL round bottom flask, and the reaction was performed under magnetic stirring at 25°C for 4 hours. Ethyl acetate was recovered by rotary evaporation under reduced pressure to obtain a yellow-brown solid residue, which was recrystallized from 95% ethanol to obtain 3.72 g of N-(2-pyrrolecarbonyl)-β-alanine methyl ester crystals, with a yield of 94.9%. The melting point is 134-135°C.
[0051] (3) Synthesis of N-(2-pyrrolecarbonyl)-β-alanine (II)
[0052] 2.94 g of N-(2-pyrroloyl)-β-aminopropionic acid methyl ester crystals, 20 mL of 15% NaOH aqueous solution, 10 mL of ethanol, stirred at room temperature for 1 hour, added dropwise 10% hydrochloric acid until slightly acidic, filtered to remove insoluble matter, The aq...
Embodiment 3
[0056] (1) Synthesis of 2-trichloroacetylpyrrole: same as Example 1
[0057] (2) Synthesis of N-(2-pyrroleformyl)-beta-alanine methyl ester (I)
[0058] 2.47g of β-alanine methyl ester, 4.25g of 2-trichloroacetylpyrrole and 20mL of ethyl acetate were added to a 50mL round bottom flask, and the reaction was performed under magnetic stirring at 35°C for 2 hours. Ethyl acetate was recovered by rotary evaporation under reduced pressure to obtain a yellow-brown solid residue, which was recrystallized from 95% ethanol to obtain 3.66 g of methyl N-(2-pyrroloyl)-β-alanine crystals, with a yield of 93.2%. The melting point is 134-135°C.
[0059] (3) Synthesis of N-(2-pyrrolecarbonyl)-β-alanine (II)
[0060] 2.94 g of N-(2-pyrrolecarbonyl)-β-aminopropionic acid methyl ester crystals, 20 mL of 10% NaOH aqueous solution, 7 mL of ethanol, stirred at room temperature for 2 hours, added dropwise 10% hydrochloric acid until slightly acidic, filtered to remove insoluble matter, The aqueous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com