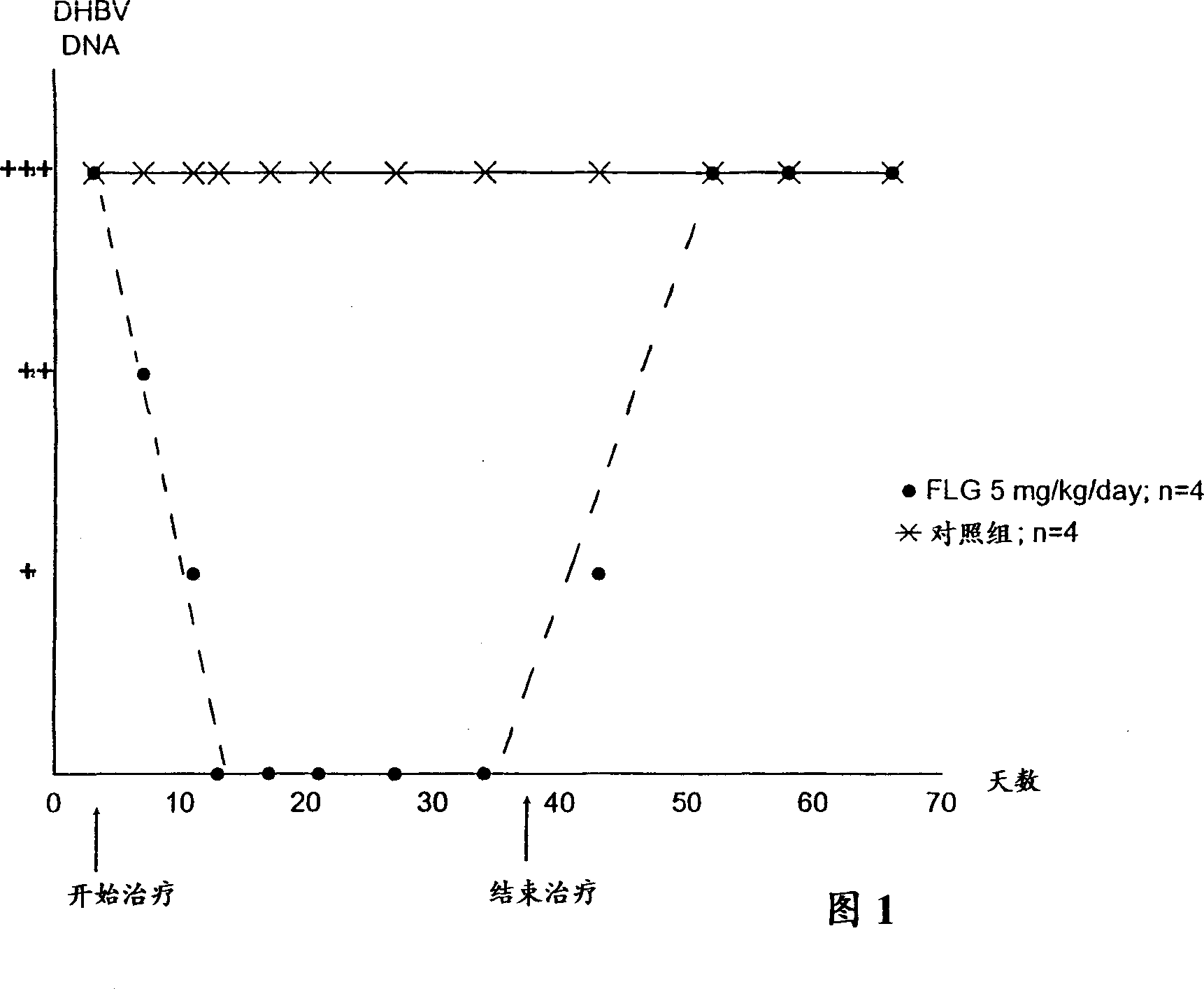
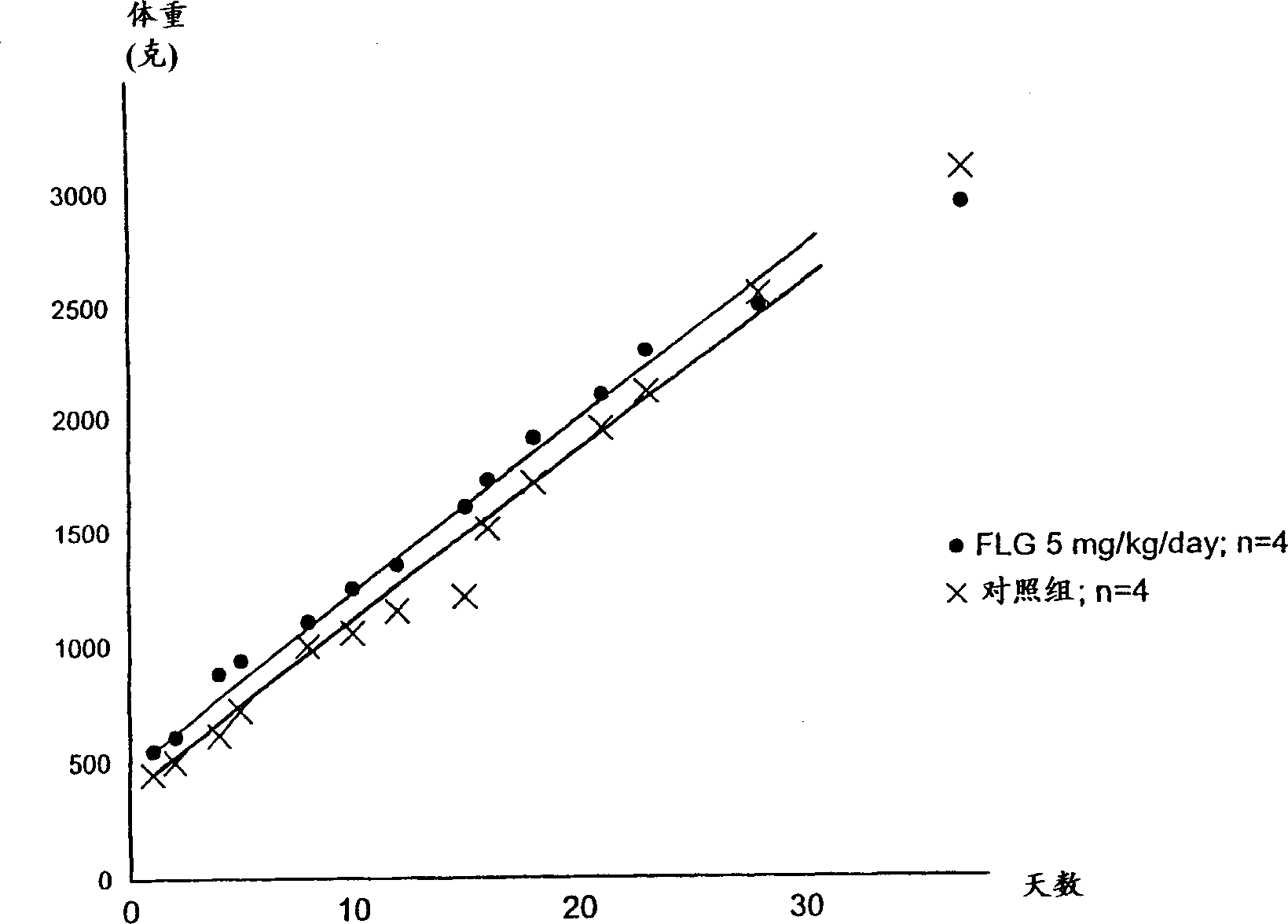
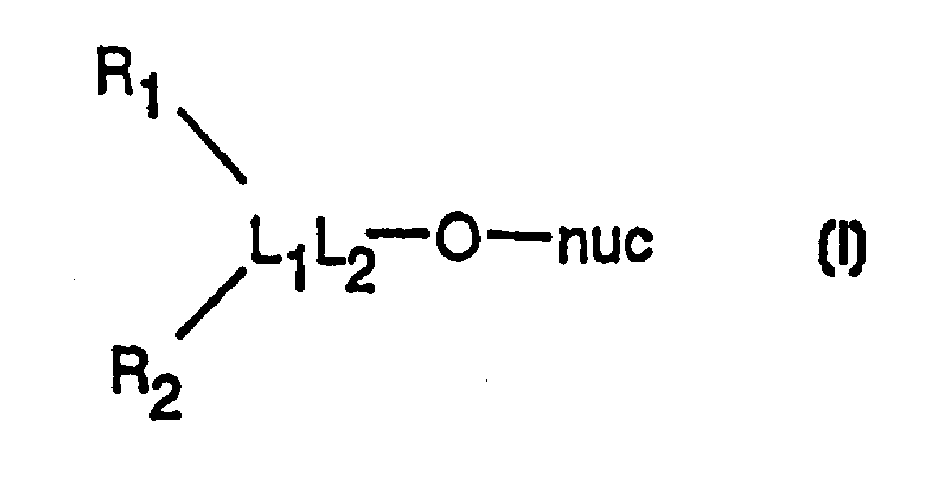Nucleosides analogues, such as antivirals including inhibitors of retroviral reverse transcriptase and the DNA polymerase of hepatitis B virus (HBV)
A technology of compounds and nucleosides, applied in the field of nucleoside analogs, can solve the problems of unreported bioavailability and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] 2-(Stearyloxymethyl)-2-(N-(fluorenylmethoxycarbonyl)-L-valyloxymethyl)-propionic acid
[0109] To a solution of 2,2-bis(hydroxymethyl)propionic acid (28.16 g, 210 mmol) in water (50 mL) was added potassium hydroxide (11.78 g, 210 mmol). After 5 minutes, the solution was evaporated in vacuo and the residue was co-evaporated three times with anhydrous DMF. The residue was then dissolved in DMF (500 mL), and to this solution was added benzoyl bromide (3.57 mL, 30 mL). After stirring for 30 minutes, the reaction mixture was filtered through Celite, poured into aqueous sodium bicarbonate and extracted with dichloromethane. The organic phases were collected and washed with an aqueous solution of sodium bicarbonate. Subsequent evaporation in vacuo gave benzyl 2,2-bis(hydroxymethyl)propionate (4.37 g), 1 H-NMR (CDCl 3 ): 7.35 (s, 5H), 5.20 (d, 2H), 3.91-3.71 (m, 4H), 1.10 (s, 3H).
[0110] To a solution of benzyl 2,2-bis(hydroxymethyl)propionate (4.37 g, 19.5 mmol) in pyri...
Embodiment 2
[0112] 2-(N-fluorenyl-methoxycarbonyl)-L-valyloxymethyl)-2-(stearyloxymethyl) in THF / methanol (16ml / 8ml) mixed solvent ) benzyl propionate (1.86 g, 3.8 mmol) was added ammonium formate (376 mg, 6 mmol), formic acid (1.87 ml) and palladium black (40 mg). The reaction was maintained at room temperature for 16 hours, then filtered through Celite. After evaporation, the product was isolated by silica gel column chromatography, yield 1.05 g. Example 21-O-stearyl-2-O-(N-CBz-L-valyl)glycerol a) Preparation of 1-O-stearylglycerol
[0113] To a mixture of glycerol (30 g, 326 mmol) and pyridine (25 mL) dissolved in DMF (300 mL) was added stearoyl chloride (10 g, 33 mmol) dissolved in DMF (100 mL) dropwise. The mixture was cooled on an ice bath until the addition was complete. Response to N 2 Keep overnight under atmosphere. CH was added after 15 hours 2 Cl 2 (300 ml) and saturated NaHCO 3 (aqueous solution). The phases were separated and the organic phase was washed with water ...
Embodiment 3
[0117] Removal of the pixyl group from the product of step d) by selective deprotection using the method described in Example 3 affords the title compound, 1 H-NMR (CDCl 3 ): δ7.35(m, 5H), 5.3-4.9(m, 4H), 4.35-4.25(m, 3H), 3.8-3.6(m, 2H), 2.31-2.25(m, 2H), 2.20-2.10 (m, 1H), 1.60 (m, 2H), 1.02-0.86 (m, 9H). Example 31-O-(N-CBz-L-valyl)-2-O-stearyl glycerol a) Preparation of 1-O-(N-CBz-L-valyl)glycerol
[0118] CBz-L-valine (4.35 g, 17.3 mmol), dicyclohexylcarbodiimide (4.29 g, 20.8 mmol) and 4-dimethylaminopyridine (0.212 g) were added together at room temperature into a five-fold excess of glycerol (8 mL, 86.9 mmol). After stirring overnight, the suspension was filtered and DMF was removed from the filtrate in vacuo. Dissolve the residue in CH 2 Cl 2 In continuous use of saturated NaHCO 3 , brine and water washes, then dried. The crude product was chromatographed on silica gel with 4 / 1 EtOAc-hexane as eluent, yield 2.465 g, Rf (4 / 1 EtOAc-hexane) 0.17, (20 / 1 CH 2 Cl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com