Compound of quinacridones-carbazole group and application in organic electroluminescence device
A technology of quinacridone and carbazole, which is applied in organic electroluminescent materials and its application fields, can solve the problems of fluorescence quenching and strict device process requirements, and achieve good stability, convenient purification, and electroluminescence efficiency high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0027] Example 1: Synthesis of TMDCzBQA
[0028] Compound 2,5-dihydroxy-1,4-cyclohexadiene-1,4-dicarboxylic acid diethyl ester 5.0g, 3,5-dimethylaniline 10ml, hydrochloric acid 0.5ml, heat to reflux with ethanol as solvent 6 hours. Cool and filter, wash the precipitate with ethanol to obtain 8.2 grams of pink product 2,5-bis(3,5-dimethylanilino)-1,4-cyclohexadiene-1,4-dicarboxylate diethyl ester, Yield 90.3%.
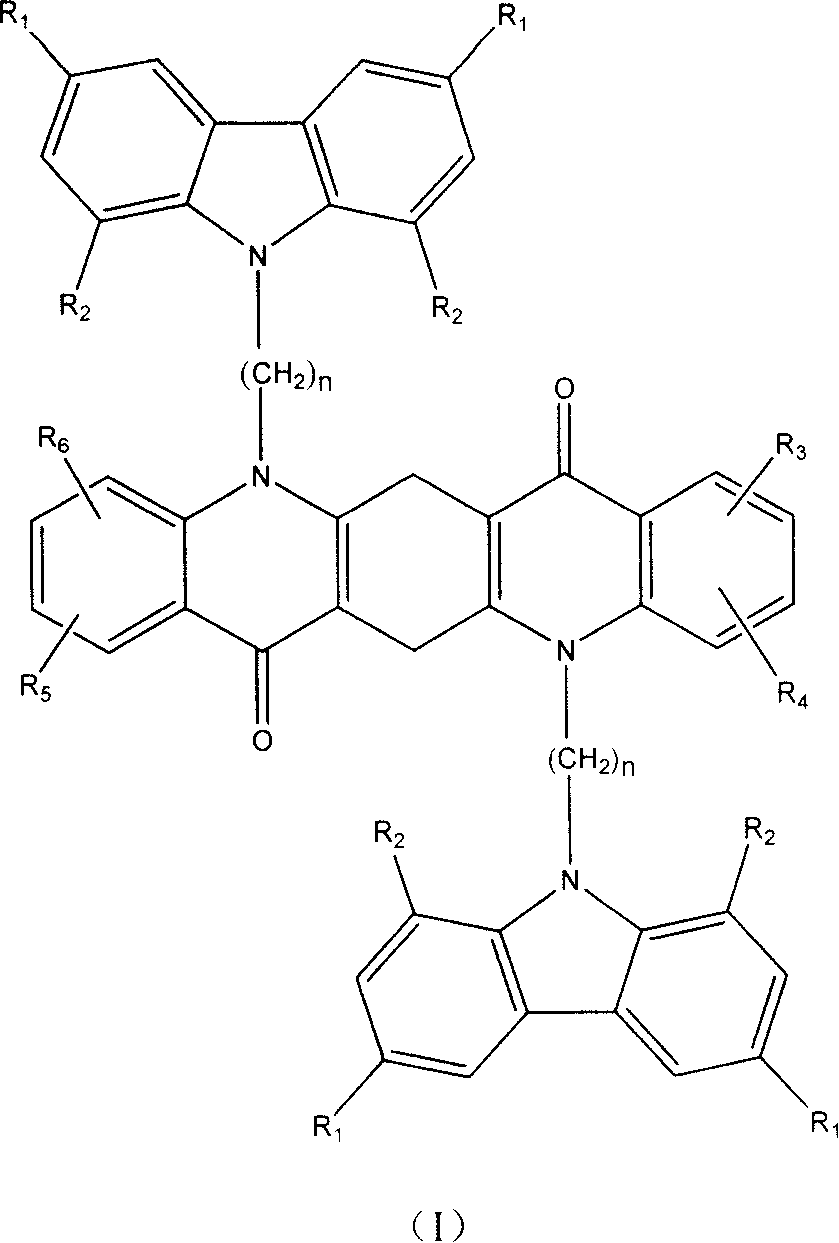
[0029] Compound 2,5-bis(3,5-dimethylanilino)-1,4-cyclohexadiene-1,4-dicarboxylate 5.0 g and 1-chloronaphthalene were heated to reflux at 260°C for 2 hours, filtered and washed with chloroform to obtain 3.2 g of the product 1,3,8,10-tetramethyl-6,13-dihydro-quinacridone with a yield of 81.2%.
[0030] Add 2.5 grams of compound 1,3,8,10-tetramethyl-6,13-dihydro-quinacridone into nitrobenzene, potassium hydroxide, and ethylene glycol monomethyl ether, heat and reflux for 2 hours, and then add 100ml of ethanol and 150ml of acetic acid were heated to reflux for 2 hours, ...
example 2
[0036] Example 2: Synthesis of TMDCzHQA
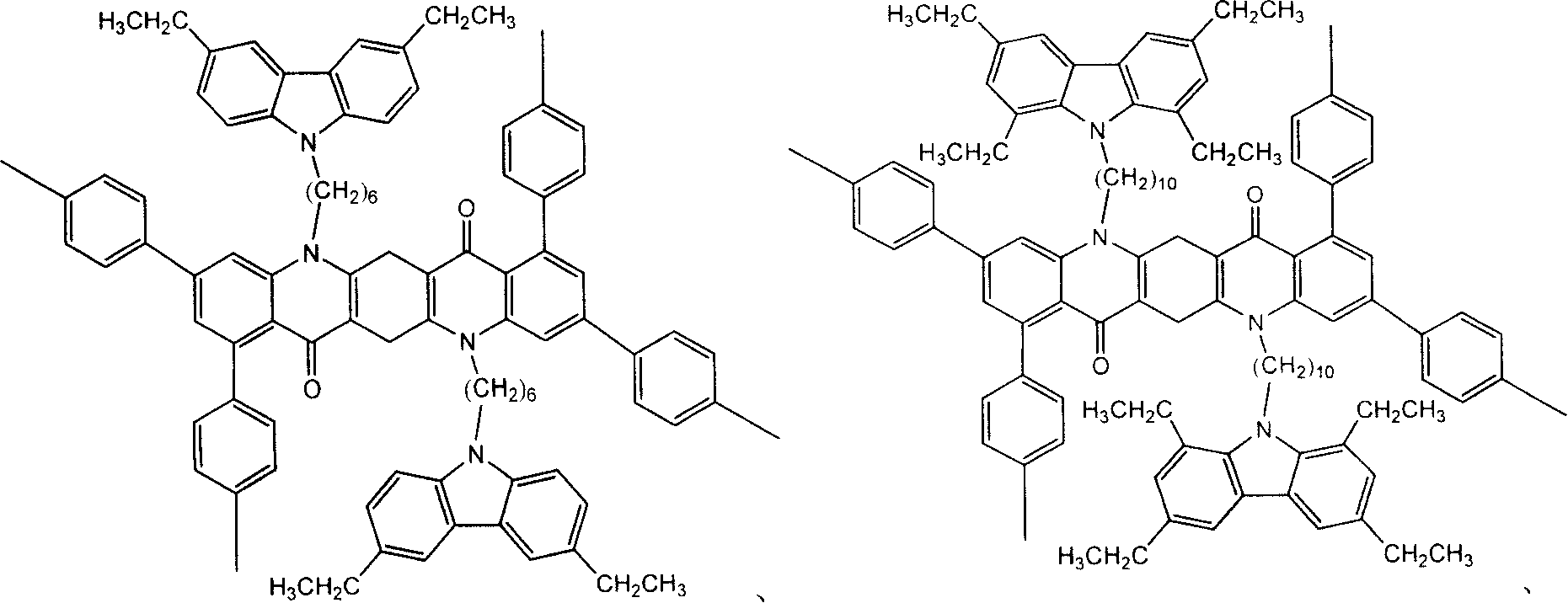
[0037] The synthesis of TMDCzHQA is the same as Example 1, just replace 1,4-dibromobutane with 1,6-dibromohexane to obtain product 1,3,8,10-tetramethyl-5,12-two {4 -N-carbazolyl)hexyl}quinacridone (TMDCzHQA). Mass spectrum molecular ion peak: 886.5. Elemental analysis according to chemical formula C 61 h 66 N 4 o 2 Calculated: C: 82.6%; H: 7.5%; N: 6.3%; found: C: 82.6%; H: 7.2%; N: 6.5%.
example 3
[0038] Example 3: Synthesis of TMDCzOQA
[0039] The synthesis of TMDCzOQA is the same as example 1, just replace 1,4-dibromobutane with 1,8-dibromooctane to obtain product 1,3,8,10-tetramethyl-5,12-two {4 -N-carbazolyl)octyl}quinacridone (TMDCzOQA). Mass spectrum molecular ion peak: 942.6. Elemental analysis according to chemical formula C 65 h 74 N 4 o 2 Calculated: C: 82.8%; H: 7.9%; N: 5.9%; found: C: 82.6%; H: 7.8%; N: 6.0%.
[0040]
[0041] 1,3,8,10-Tetramethyl-5,12-bis- 1,3,8,10-Tetramethyl-5,12-bis-(4-N-carbazolyl)-hexyl)quinacridone (4-N-carbazolyl- octyl)quinacridone
[0042] TMDCzHQA TMDCzOQA
PUM
| Property | Measurement | Unit |
|---|---|---|
| Shine | aaaaa | aaaaa |
| Shine | aaaaa | aaaaa |
| Shine | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com