Heteropolycyclic compounds and their use as metabotropic glutamate receptor antagonists
A compound and composition technology, applied in anti-inflammatory agents, metabolic diseases, non-central analgesics, etc., can solve obstacles to mGluR physiology, pharmacology and treatment, limited value, lack of efficacy and selection of mGluR agonists and antagonists sexual issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
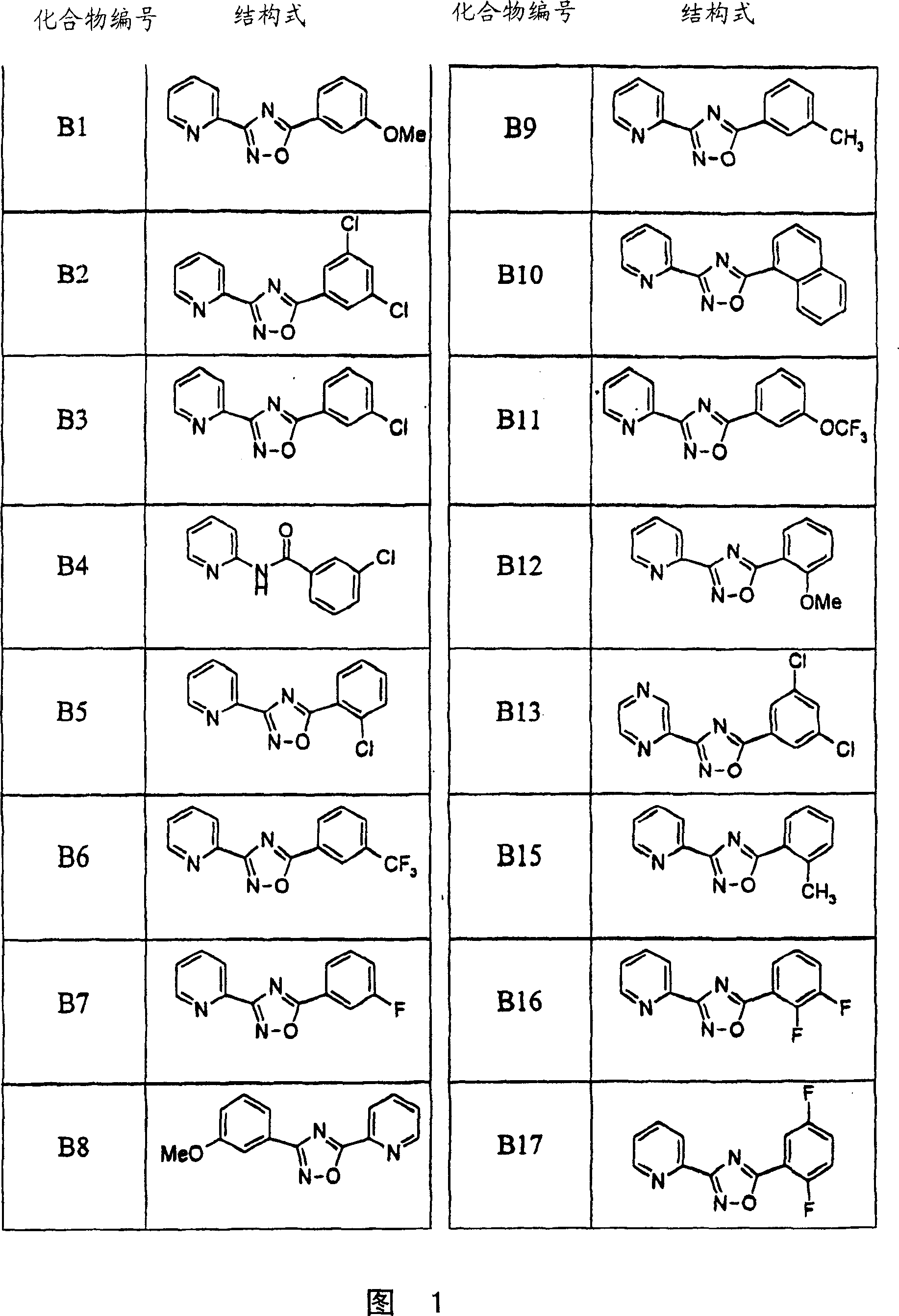
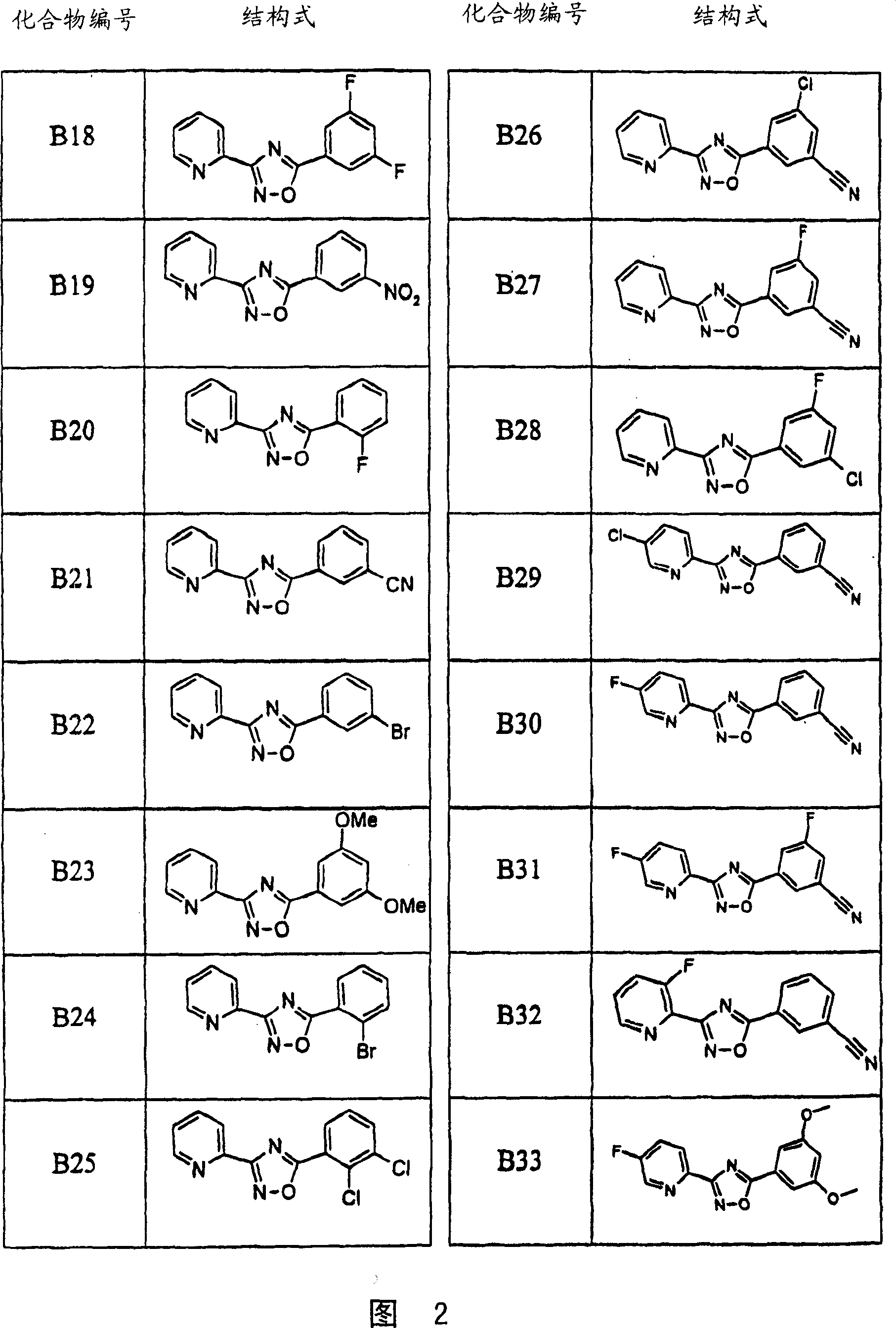
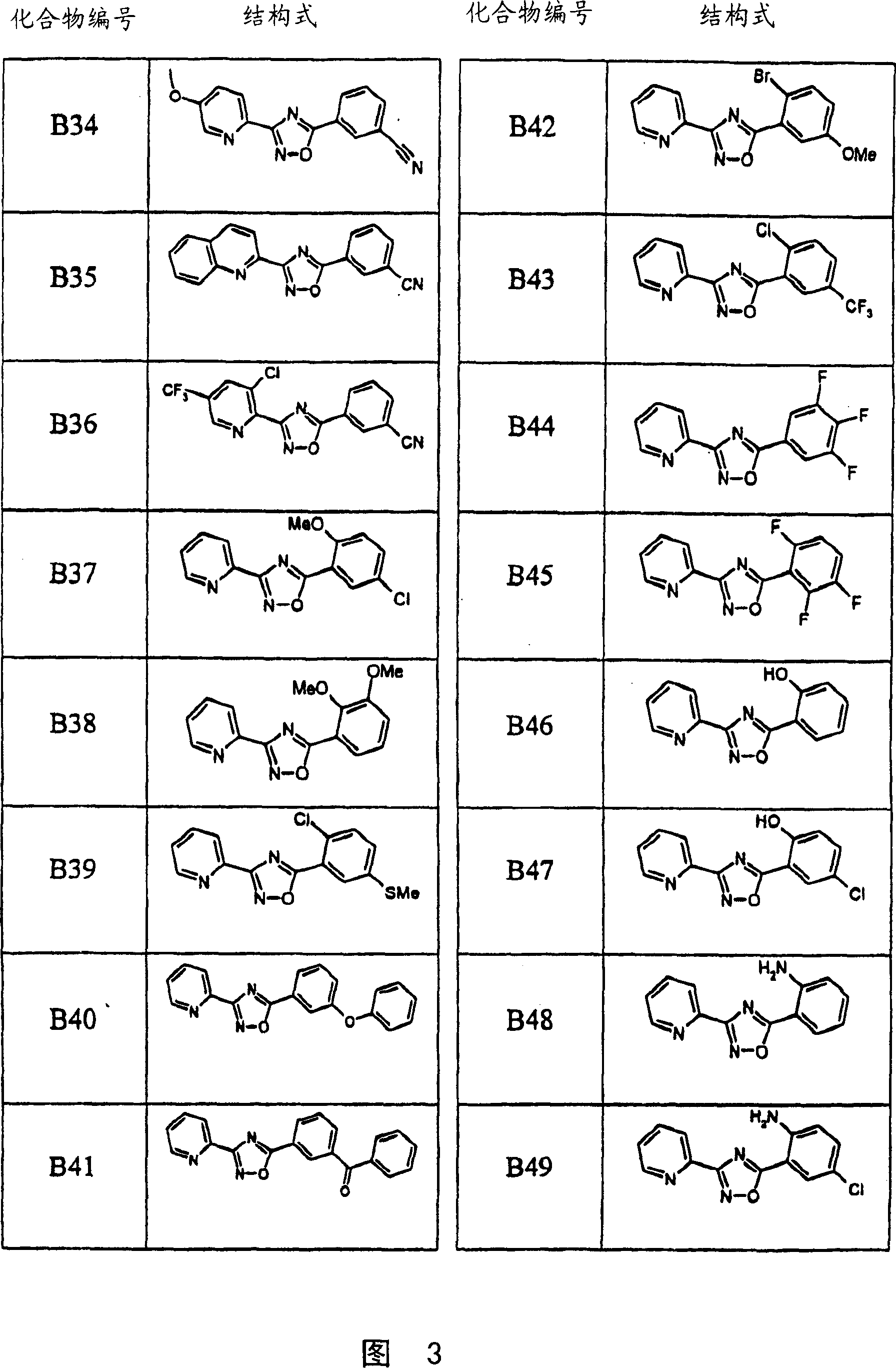
Image
Examples
Embodiment 1
[0093] Embodiment 1: synthetic amidoxime intermediate
[0094] Pyridin-2-ylamidoxime
[0095]
[0096] Using the method of Shine et al., J.Heterocyclic Chem. (1989) 26:125-128, hydroxylamine hydrochloride (7.65g, 110mmol) in ethanol (100mL) was dissolved in sodium hydroxide solution (11mL, 10N, 110mmol )deal with. A precipitate formed rapidly and the reaction mixture was stirred at room temperature for 30 minutes. The inorganic precipitate was filtered off and washed with ethanol (100 mL). The filtrate was combined with the ethanol washes and treated with 2-cyanopyridine (10.4 g, 100 mmol). The reaction mixture was heated to reflux for 20 hours. The volatiles were then removed in vacuo to afford 13.3 g (97%) of pyridin-2-ylamidoxime.
[0097] 3-Methoxybenzamide oxime
[0098]
[0099] Using the general procedure for the synthesis of amidoximes, hydroxylamine hydrochloride (7.65 g, 110 mmol), sodium hydroxide (11 mL, 10 N,...
Embodiment 2
[0120] Embodiment 2: synthetic carboxylic acid intermediate
[0121] 3-Chloro-5-cyanobenzoic acid
[0122]
[0123] Methyl 3,5-dichlorobenzoate (14.66g, 71.5mmol), zinc cyanide (5.04g, 42.9mmol), zinc (zinc powder, 0.21g, 3.21mmol), [1,1′-di( Diphenylphosphino) ferrocene] dichloropalladium (II) complex with dichloromethane (1:1) (1.3g, 1.57mmol) in N,N-dimethylformamide (70mL) The mixture was heated to reflux for 5 hours. After cooling, the reaction was diluted with ethyl acetate and extracted with water and brine. Purification by silica gel chromatography afforded 2.34 g (17%) of methyl 2-chloro-5-cyanobenzoate. The intermediate ester was treated with sodium hydroxide solution (7.5 ml, 4N solution, 30 mmol) in methanol (50 mL) and stirred at room temperature for 18 hours. The solvent was removed in vacuo, and the residue was dissolved in ethyl acetate. The organic solution was washed with 5% HCl and brine. Removal of the solvent afforded 1.8 g (83%) ...
Embodiment 3
[0132] The crude methyl 3-chloro-5-cyanobenzoate was treated with sodium hydroxide solution (45 ml, 4N solution, 180 mmol) in methanol (350 mL) at room temperature for 4 hours. The solvent was removed in vacuo, and the residue was dissolved in ethyl acetate. The organic solution was washed with 5% hydrochloric acid and brine. Purification by silica gel chromatography afforded 7.0 g (54%) of 3-fluoro-5-cyanobenzoic acid. Example 3: Synthesis of 3,5-disubstituted-1,2,4-oxadiazoles from acid chlorides
[0133] Generally a modification of the method given by Shine et al. in J. Heterocyclic Chem. (1989) 26: 125-128 is used. 3,5-disubstituted-1,2,4-oxadiazoles are usually prepared by adding the acid chloride to a solution of amidoxime in pyridine, then heating the reaction mixture to reflux, or placing it in a sealed tube and heat. Oxadiazoles are generally isolated by precipitation with cold water and filtration or extraction with organic solvents. Purify the oxadiazole by chr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com