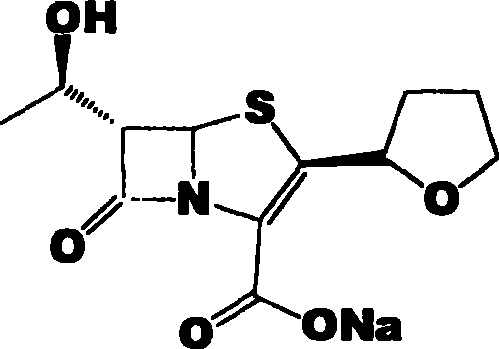
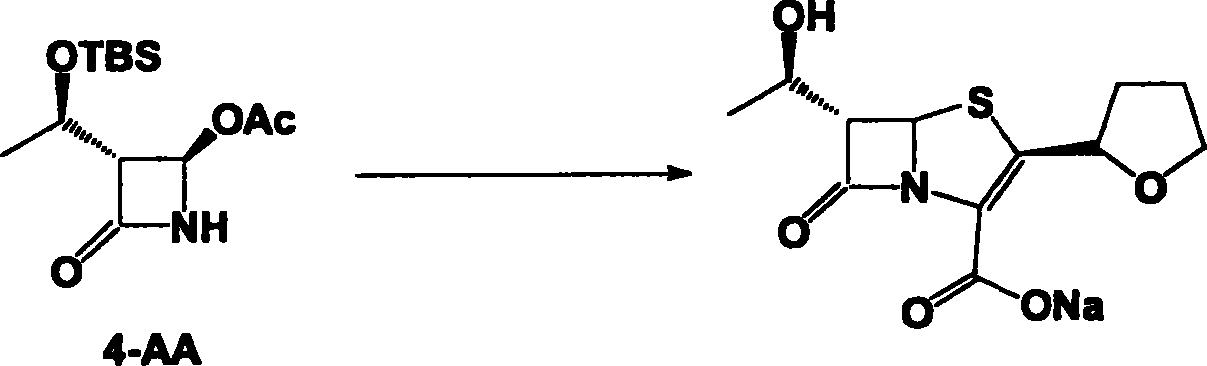
Faropenem sodium preparation method
A synthesis method and intermediate technology are applied in the field of preparation of faropenem sodium, and can solve the problems of difficulty in realizing large-scale industrial production of faropenem sodium, low yield of faropenem sodium, long process route, etc., and the reaction conditions are easy to realize, The effect of high yield and strong controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 240g (R)-tetrahydrofuran-2-thioformic acid and 490g 4-AA into a 2000mL three-neck flask, add 1200mL ethyl acetate to it, and slowly add about 400mL 4N NaOH dropwise after dissolving to adjust the pH to 12-13 , and the reaction was stirred until the reaction was complete. Extract with 1500 mL ethyl acetate, collect the organic phase, wash with brine, and dry over anhydrous sodium sulfate. The condensate (440 g of colorless viscous liquid) was obtained by spin-drying under reduced pressure, which was directly used in the next step.
Embodiment 2
[0027] The condensate obtained in Example 1 was dissolved in 2000mL of dichloromethane, placed in a 3000mL three-necked flask, and 453.0mL of pyridine was added, cooled to 0°C in an ice-water bath, and 350g of allyloxyfen was added dropwise at 5-15°C A mixture of acid chloride and 350 mL of dichloromethane. After the dropwise addition is complete, continue to react under this condition for about 30 minutes. The plate layer monitors that the reaction is complete. Add 1500mL of deionized water, and the organic phase is washed with 1500mL of deionized water, 1500mL of saturated sodium bicarbonate solution, and then washed with 1500mL of saturated brine. Dry over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain the condensate (580 g of colorless solid), which was set aside.
Embodiment 3
[0029] Dissolve 250 g of the solid obtained in Example 2 in 6000 mL of xylene, add 25 g of triethylamine, add it to a 10000 mL three-necked flask, stir mechanically, heat to reflux with an electric heating mantle, and add 655 mL of triethyl phosphite dropwise, about 120 Minutes dropwise completed. Heat preservation and reflux reaction for 16 hours, and concentrate under reduced pressure to obtain the ring compound (yellow solid 170 g), which is set aside.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com