Aqueous immunologic adjuvant compositions of monophosphoryl lipid a
A monophosphoryl lipid and composition technology, applied in the direction of drug combination, sugar derivatives, sugar derivatives, etc., can solve the problems of reducing the effectiveness of ingredients, stimulating the mucosal surface, inflammation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The modified 3D-MLA used in the present invention is prepared by base hydrolysis of MLA under conditions that result in the loss of a single fatty acid at position 3 of the lipid A backbone. In alkaline media, the β-hydroxymyristyl fatty acid at position 3 is unusually unstable. Lipid A can be completely 3-deacylated only by mild alkali treatment. The other lipid A ester bonds require some more intense conditions before hydrolysis occurs so as to selectively deacylate the moiety at position 3 without significantly affecting the rest of the molecule. The reason for the unusual sensitivity of ester-linked β-hydroxymyristyl fatty acids at position 3 to alkaline media is currently unknown.
[0019] Although basic hydrolysis methods are known, it is important to choose conditions that do not cause further hydrolysis other than hydrolyzing the ester bond of the β-hydroxymyristyl group at position 3. Typically hydrolysis can be carried out in aqueous or organic media. In th...
Embodiment 1
[0029] Example 1 - Preparation of Aqueous Formulations of Attenuated Lipid A Derivatives
[0030] According to the present invention, water for injection contained 1000 μg / ml of the attenuated lipid A form of 3D-MLA from Salmonella minnesota R595 (RiBi ImmunoChem Research, Inc., Hamilton, Montana 59840) and 118 μg / ml of 1,2-bis An aqueous formulation of 3-O-deacylated monophosphoryl lipid A (3D-MLA / AF) of palmitoyl-sn-glycero-3-phosphocholine (DPPC) was prepared as follows:
[0031] DPPC was dissolved in ethanol to a concentration of 4 mg / ml and swirled to clarify. Add 2.7 ml of DPPC solution to the vial containing 100 mg lyophilized 3D-MLA and swirl gently to wet the 3D-MLA. Gently blow filtered nitrogen into the vial to remove ethanol. Water for injection (91.7ml) was added to the vial, then the vial was stoppered, sealed and suspended in a Labline 9303 water bath ultrasonicator. The suspension was sonicated at 60°C for 10 minutes until clear. The resulting aqueous form...
Embodiment 2
[0032] Example 2 - Stimulation of antibody responses
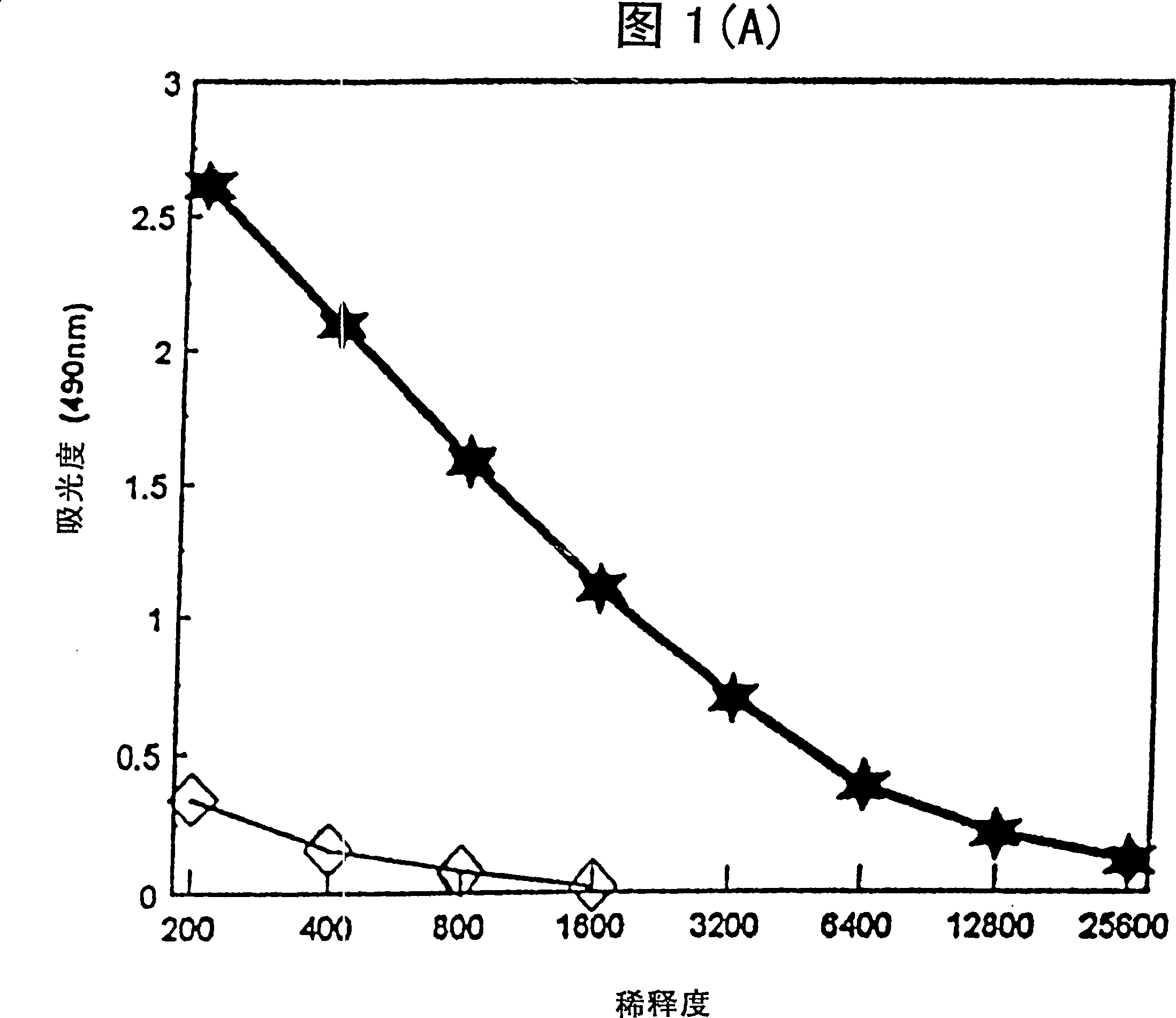
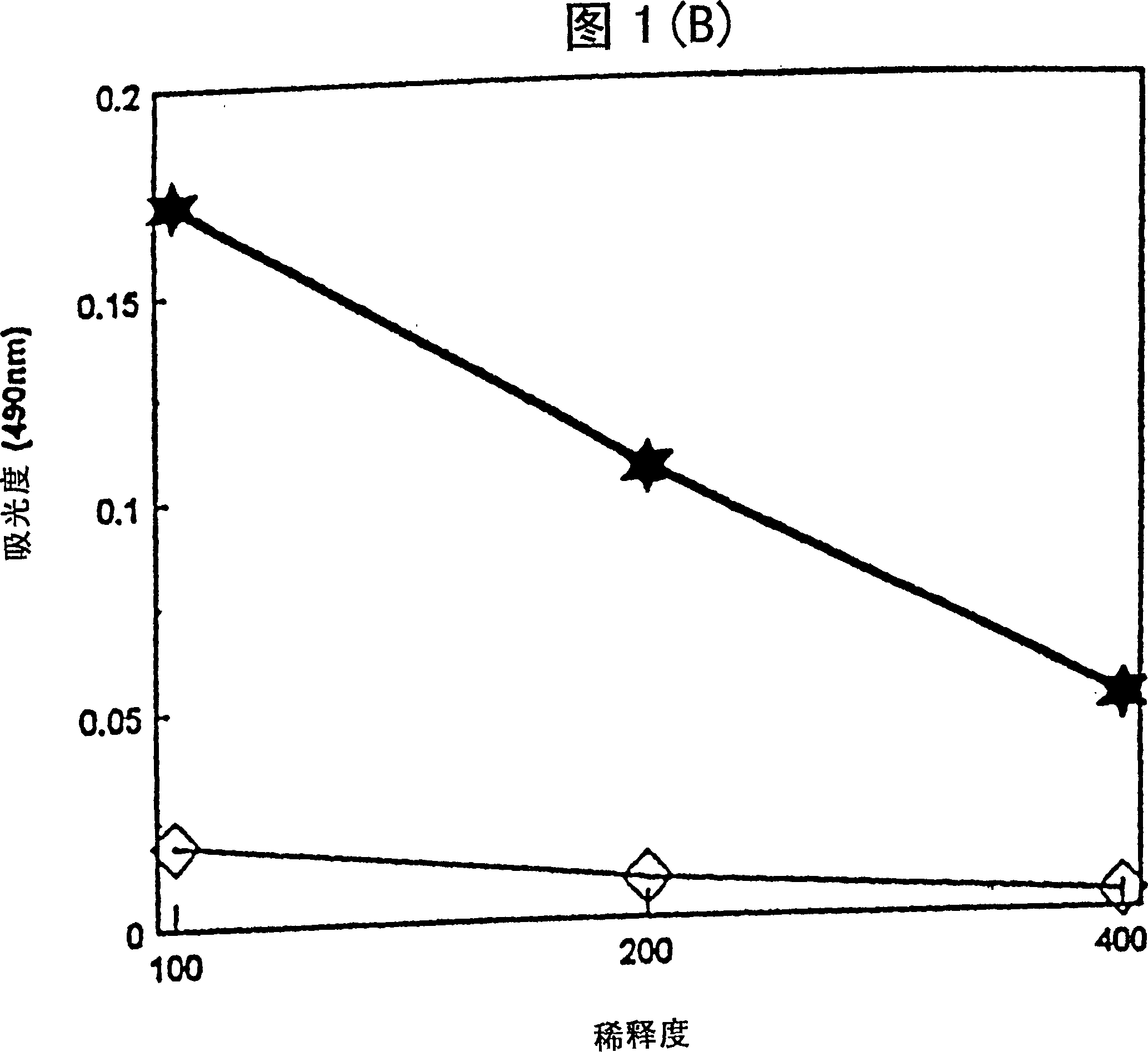
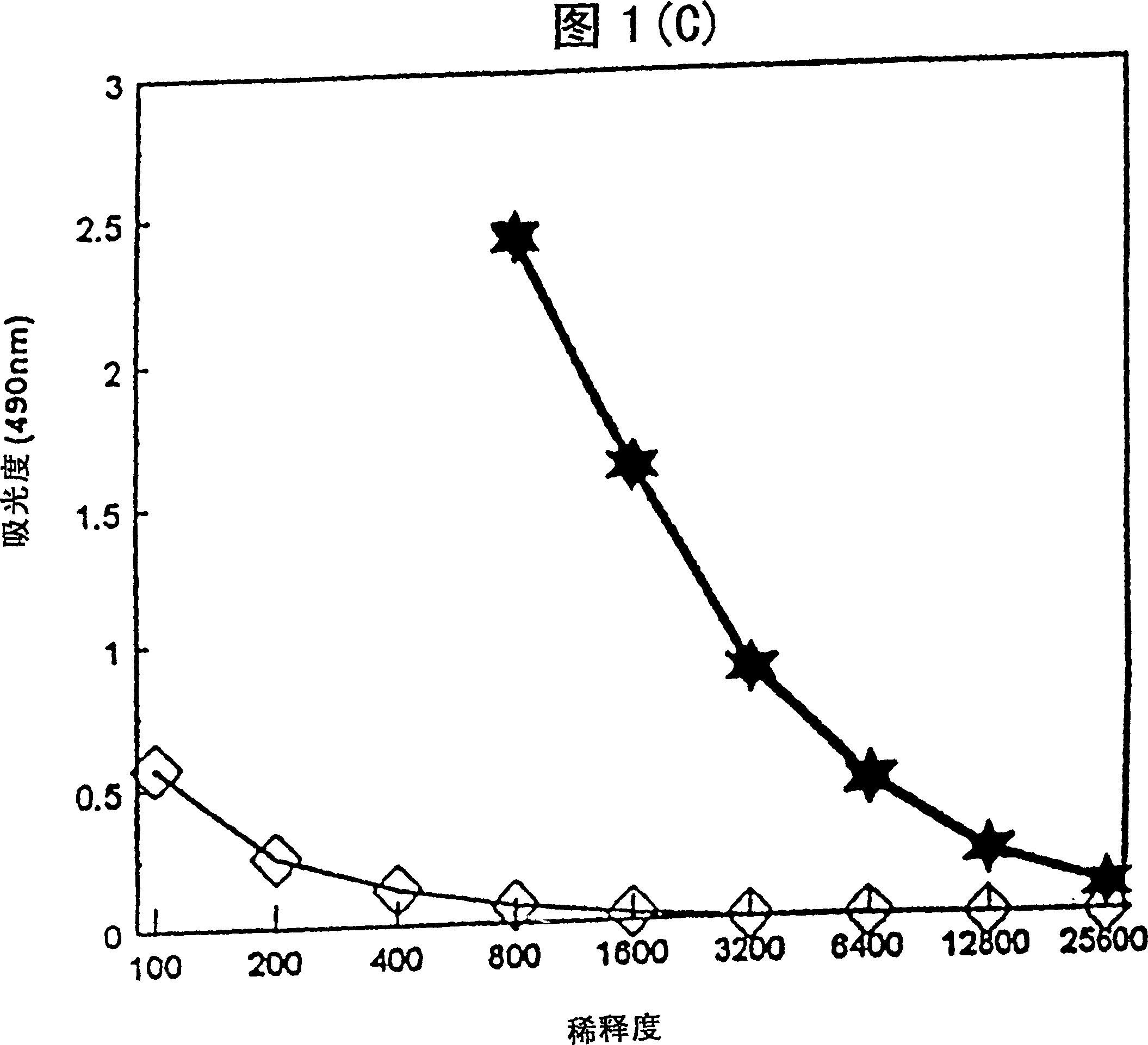
[0033] Mice immunized with tetanus toxoid (TT) in the aqueous formulation of the present invention produce tetanus toxoid-specific antibodies. After immunization, TT-specific total IgG titers and IgG isotype (2a, 2b, 1) titers in mouse sera were determined by enzyme-linked immunosorbent assay (ELISA).
[0034] Female ICR mice were immunized with a dose of vaccine containing 0.1 μg tetanus toxoid (TT) + 50 μg 3D-MLA / AF or 0.1 μg TT with saline. 3D-MLA / AF was prepared according to Example 1. Vaccines were administered subcutaneously on days 0 and 21. Sera were collected 14 days after the second immunization and assayed by standard ELISA technique to report tetanus toxoid-specific antibody IgG 1 , IgG 2a and IgG 2b Relative amounts of isotypes and total IgG.
[0035] Figure 1 shows the titers of tetanus toxoid-specific antibodies produced by 3D-MLA / AF. 3D-MLA / AF stimulates IgG antibody production in immunized animals...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com