Mammalian transgenesis by intracytoplasmic sperm injection
A technology of mammals and sperm, applied in the direction of plant genetic improvement, genetic engineering, micro-injection, etc., can solve the problem of unreliable ability of transgenic animals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
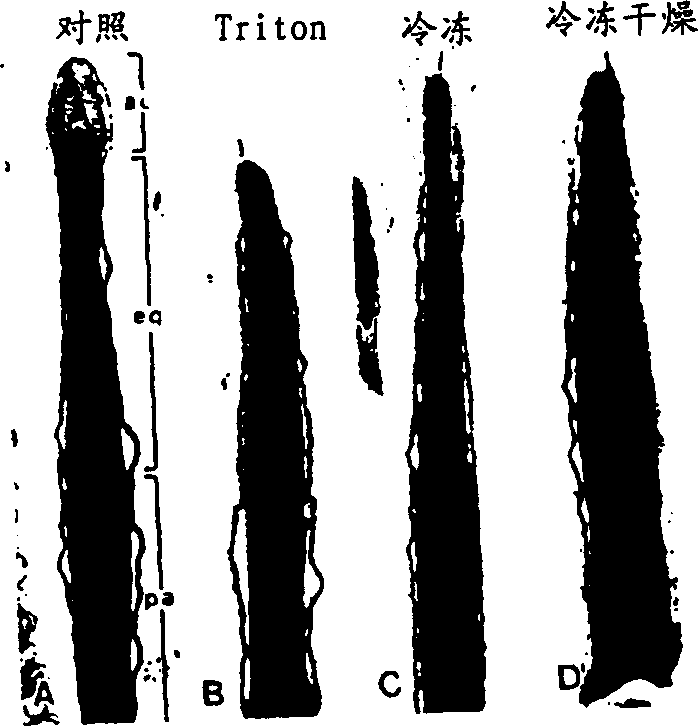
[0020] Preparation of Fresh Sperm
[0021] Fresh spermatozoa from invertebrates and vertebrates can be collected by methods known to those skilled in the art. For example, mature sperm of rodents (such as mice, golden (Syrian) hamsters, guinea pigs), rabbits, etc. can be collected from the cauda epididymis; Here, mature spermatozoa can be isolated from the freshly ejaculated semen of fertile males. Sperm from fish (eg swordtail, Xiphophorus helleri) and invertebrates such as sea urchin (Tripneustes gratilla) can be collected from the testes of mature males.
[0022] Below is an example of a method for obtaining sperm from the cauda epididymis. The caudate epididymis of mature male mice (approximately 8 weeks or older after birth) was removed. Remove blood and adipose tissue from the surface of the cauda epididymis. It is then compressed, releasing a dense ball of sperm. Place 1 drop (about 2 microliters) of sperm pellet at the bottom of a 1.5ml polypropylene centrifuge tu...
Embodiment 1
[0090] Oocyte Preparation
[0091] Maturation of B6D2F1(C57BL / 6×DBA / 2) was induced by sequential (48 h apart) injections of 7.5 international units (IU) of pregnant mare serum gonadotropin and 7.5 IU of human chorionic gonadotropin (hCG) Female mice superovulate. 14 hours after hCG injection, cumulus-oocyte complexes were collected from oviducts and treated with Hepes-CZB medium supplemented with bovine testicular hyaluronidase (300 USP units / mL, ICN Biochemicals, Costa Mesa, CA) for 3 min , to disperse the cumulus cells. Before injection of sperm nuclei, oocytes were washed and incubated in 5% (v / v) CO 2 Store in CZB medium under mineral oil equilibrated at 37°C in air for up to 4 hours.
Embodiment 2
[0093] Preparation of Fresh Sperm
[0094] Freshly collected sperm from the cauda epididymis of B6D2F1 male mice. Squeeze each epididymis with a finger while piercing its distal portion with sharp forceps. Transfer the dense semen mass exuded from the epididymis to a Petri dish. A few drops (approximately 2 microliters) of sperm were placed at the bottom of a 1.5 milliliter polypropylene microcentrifuge tube (Fisher Scientific, Pittsburgh, PA) and overlaid with 0.2-0.5 milliliters of CZB medium. After approximately 20 minutes of incubation, the upper 0.4 ml of medium was collected and examined. More than 90% of the sperm in this suspension (approximately 3-10×10 6 ) can swim lively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com