Ultra-fine particles of zinc oxide, method for preparing the same and cosmetic comprising the same
A technology of ultra-fine particles and manufacturing methods, which is applied in the direction of zinc oxide/zinc hydroxide, cosmetics, cosmetics, etc., can solve the problems of particle size, uneven particle size, decreased purity, high cost, etc., and achieve high light transmittance and whiteness , the effect of good characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
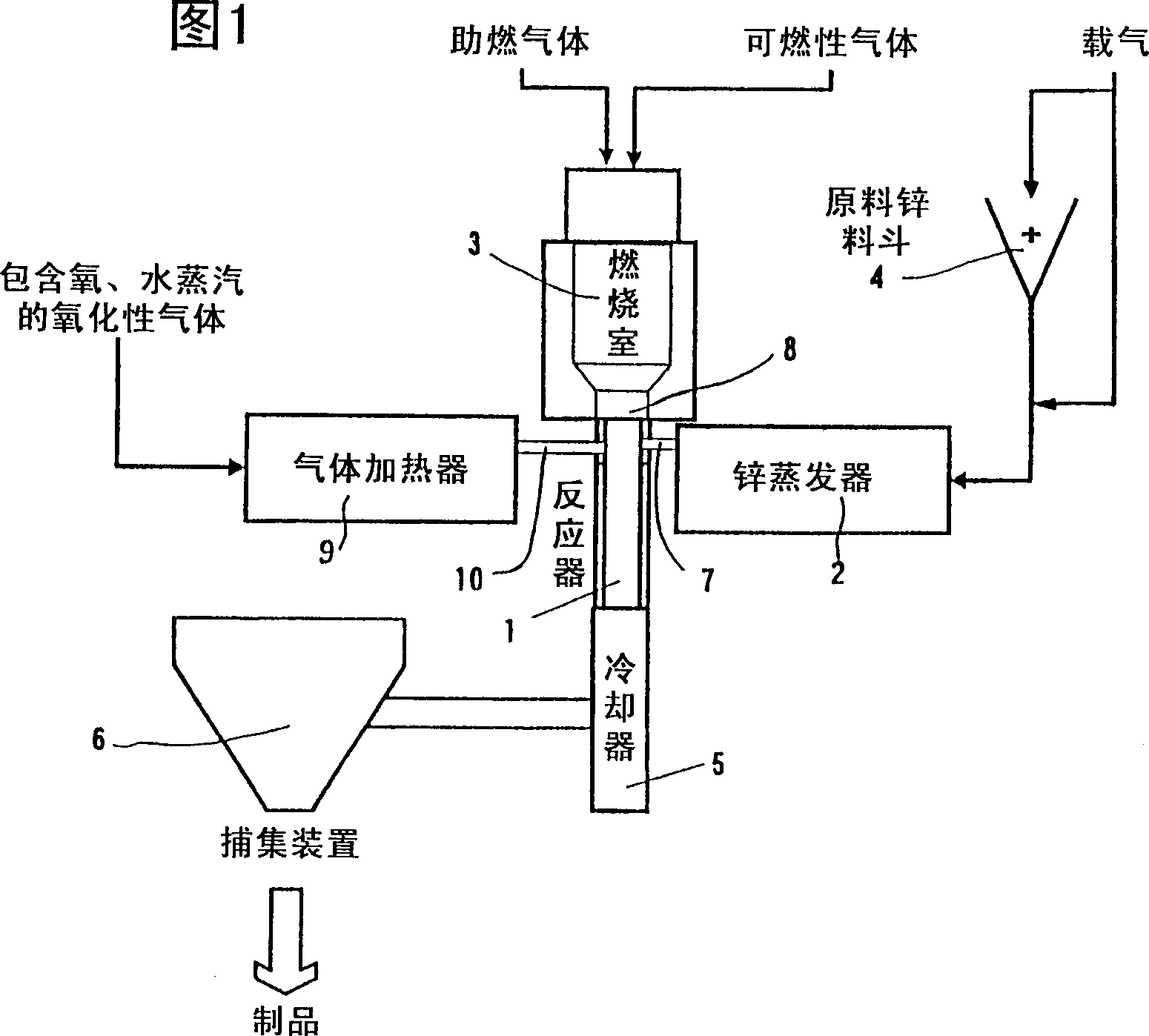
[0060] Ultrafine particle zinc oxide was produced using the reaction apparatus shown in FIG. 1 above (the same applies to Examples 2 to 4 described later).
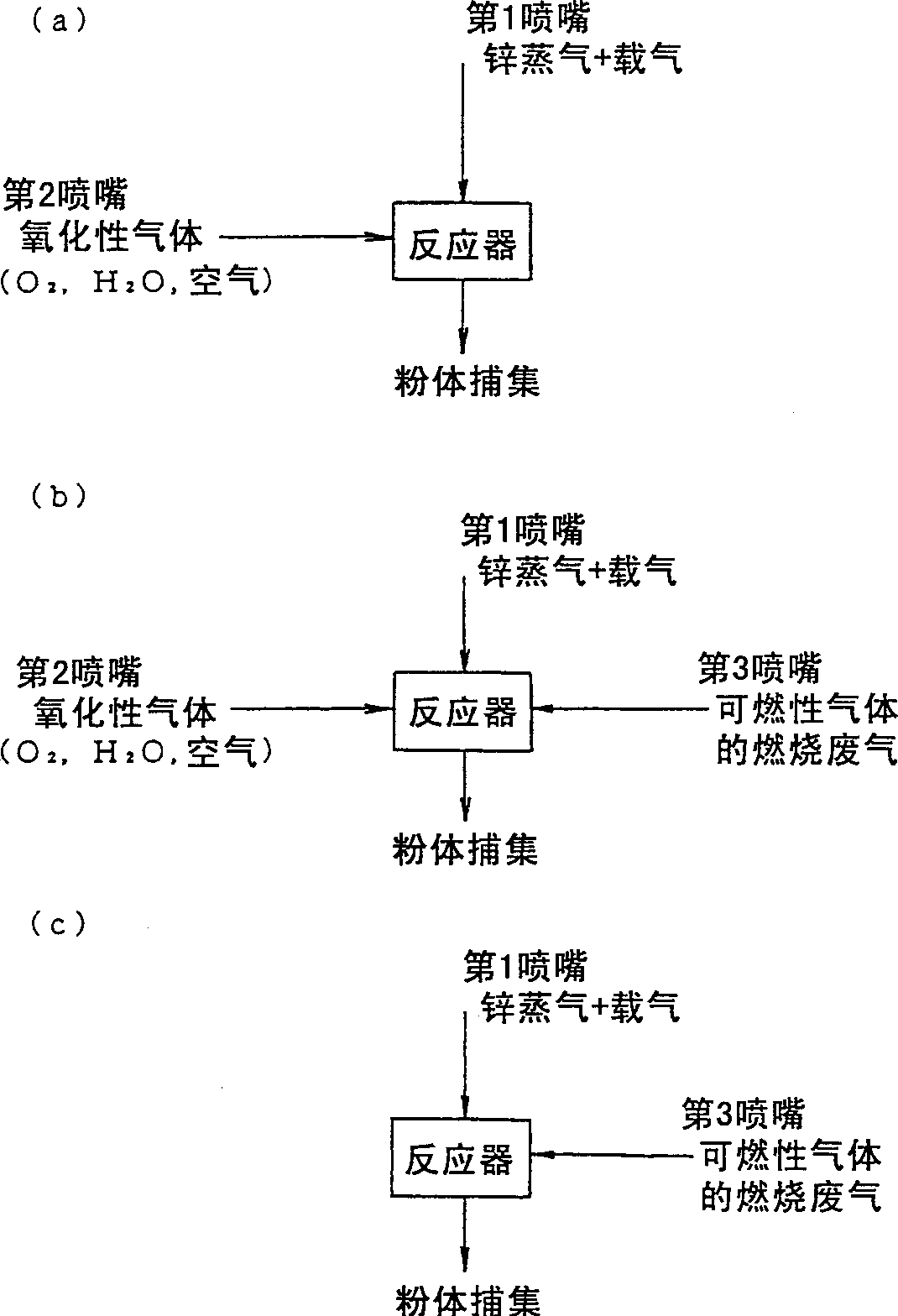
[0061] Heat a container with a large heat transfer area that can obtain a metal zinc evaporation of 9kg / hr to 1150°C, and use 4Nm of nitrogen as a carrier gas 3 / hr was blown into it, and it was introduced from the nozzle 1 to the reaction tube in a state of keeping warm. On the other hand, the air flow rate of 20Nm 3 / hr, 700cc / hr of water was heated to 1100°C, introduced into the reaction tube from the nozzle 2, and reacted with the above-mentioned raw material gas. When the white powder thus obtained was analyzed by X-ray diffraction, it was found to be Zincite, and it was confirmed to be zinc oxide. The detailed fabrication conditions are shown in Table 1.
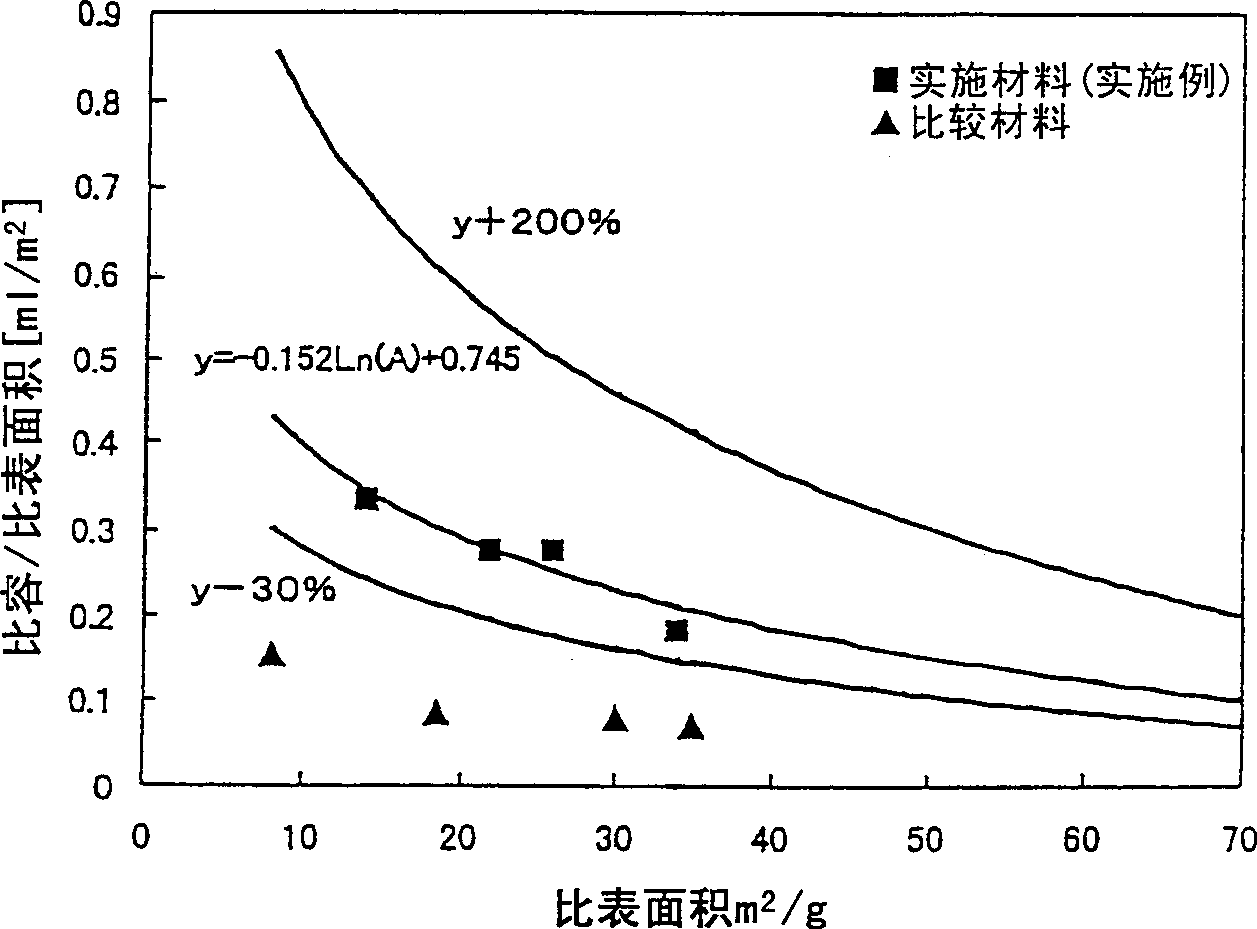
[0062] Table 2 shows the physical properties of the ultrafine particle zinc oxide thus obtained (implementation material 1).
Embodiment 2
[0064] A vessel with a large heat transfer area to obtain a metallic zinc evaporation of 4 kg / hr was heated to 1100°C. Nitrogen 1Nm as carrier gas 3 / hr was blown into it, and it was introduced from the nozzle 1 to the reaction tube in a state of keeping warm. On the other hand, the oxygen flow rate is 40Nm 3 / hr, 6 liters / hr of water was heated to 1100°C, introduced into the reaction tube from the nozzle 2, and reacted with the above-mentioned raw material gas. When the white powder thus obtained was analyzed by X-ray diffraction, it was found to be Zincite, and it was confirmed to be zinc oxide. The detailed fabrication conditions are shown in Table 1.
[0065] Table 2 shows the physical properties of the ultrafine particle zinc oxide thus obtained (implementation material 2).
Embodiment 3
[0067] A vessel with a large heat transfer area capable of obtaining a metallic zinc evaporation of 10 kg / hr was heated to 1100°C. Nitrogen 2Nm as carrier gas 3 / hr was blown into it, and it was introduced from the nozzle 1 to the reaction tube in a state of keeping warm. On the other hand, the oxygen flow rate of 120Nm 3 / hr, 15 liters / hr of water was heated to 1150°C, introduced into the reaction tube from the nozzle 2, and reacted with the above-mentioned raw material gas. When the white powder thus obtained was analyzed by X-ray diffraction, it was found to be Zincite, and it was confirmed to be zinc oxide. The detailed fabrication conditions are shown in Table 1.
[0068] Table 2 shows the physical properties of the ultrafine particle zinc oxide thus obtained (implementation material 3).
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com