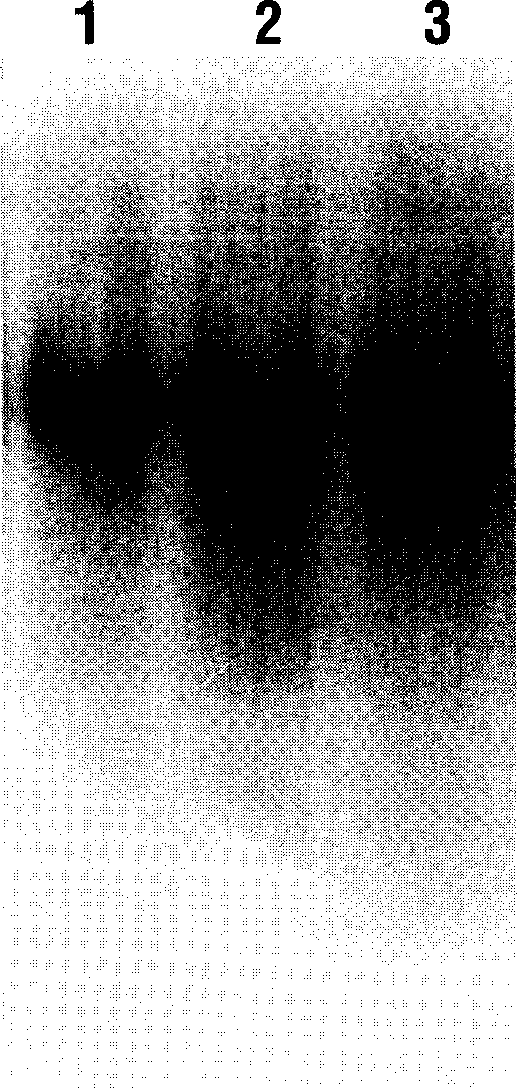Tandem immuno-assay for cancer
A technology of immunoassay and assay method, applied in immunoglobulin, anti-enzyme immunoglobulin, microbial assay/inspection, etc., and can solve problems such as non-specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0060] Example 1: Isolation and purification of cell fluid NDP-Kinase from HL60 cells
[0061] The method for purifying cytosol NDP-Kinase from HL60 cells is described by Pulido-Cejudo et al. (FASEB J3: 608a, 1989). Briefly, this cell extract fraction was obtained from washing HL60 cell microparticles with 15 g (wet weight) of PBS buffer and applying it to a phosphate glucose column (P11) (1.6 cm x 28.0 cm), which had been previously buffered with phosphate buffer. solution (50mM potassium phosphate pH7.0, 2mM MgCl 2 , 1mMPMSF, 1mMDTT and 10% glycerol). Balanced. Wash with 300ml of the same buffer at a flow rate of 0.1ml / min. Unbound protein was concentrated into 10 ml by high-speed centrifugation, and YM5 membrane (5000 M.W. cutoff, AmiconDiv., Danvers, MA., USA) was used for high-speed centrifugation. The concentrate was added to a DEAE dextran column (2.6cm×28.5cm), which was pre-washed with Tris-buffer (50mM Tris-HCl pH7.5; 2Mm MgCl 2 ; 1 mM PMSF; 1 mM DTT and 10% (v / ...
example 2
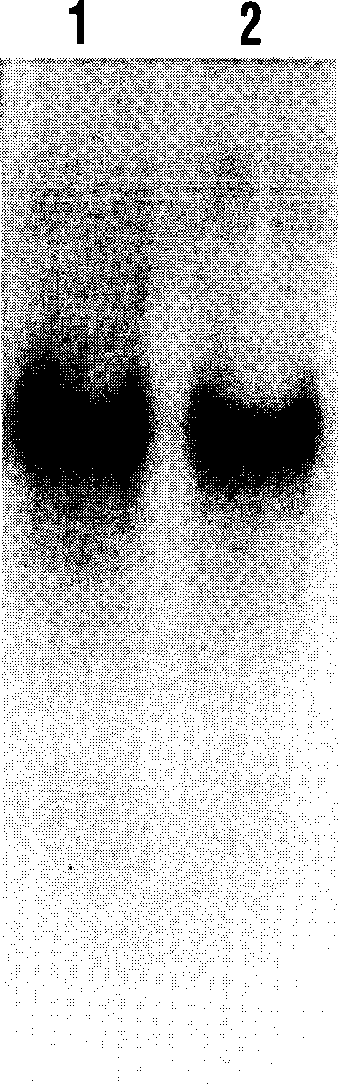
[0066] Example 2: Separation and purification of es-LAPase
[0067]Breast cancer blasts from human tumor tissue were stimulated with 100nm 17-B-Estradiol for 24 hours, or culture medium was used as control. The cell medium is: RPMI 1640 culture medium + 10% FCS + 100U / ml penicillin + 100ug / ml streptomycin. Supernatants were collected after digestion with PBS in seamless dextran tubes (MV 12,400) for 24 hours (4°C). LAP was then purified from the degraded cell supernatant using HPLC-gel permeation chromatography with DEAE-cellulose and Bestatin-Sepharose affinity chromatography. Briefly, the cell supernatant was poured onto a Bio-Sil SEC-250 column (600×7.5 mm) containing 100 mM sodium phosphate (pH 6.8), 100 mM Na 2 SO 4 , 1uM ZnCl 2 , and 10% glycerol buffer pre-equilibrated. The column was then washed with 300ml of the same buffer at a flow rate of 0.5ml / min. The protein was concentrated to 10ml by high-speed centrifugation with YM5 membrane. The concentrated solution...
example 3
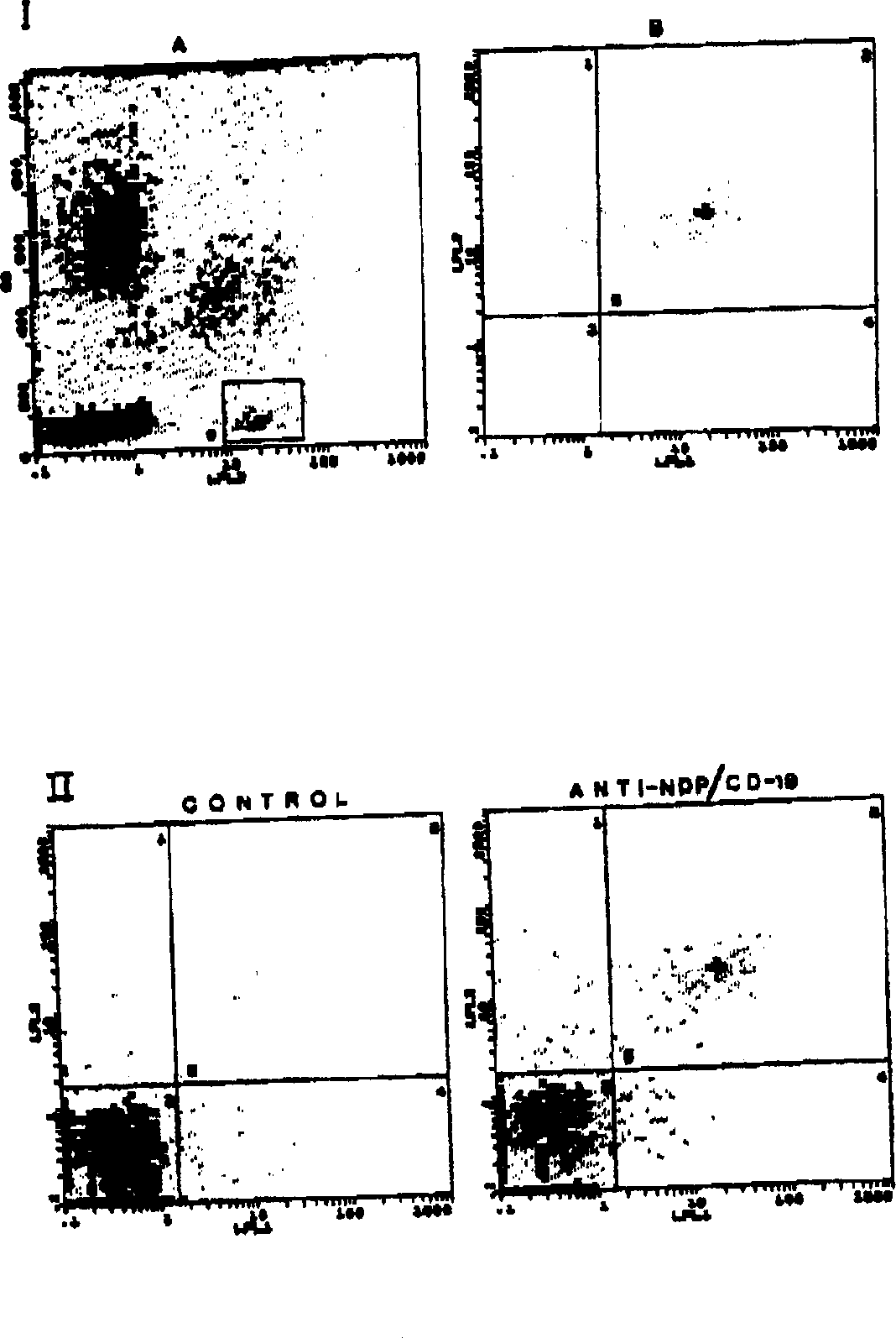
[0068] Example 3: Production and purification of monoclonal antibodies MAb 7B6 (anti-LAPase) and MAb 4A12 (anti-NDP-Kinase)
[0069] Methods in this protocol such as the preparation of immunizing antigens, the preparation of spleen cells from immunized animals, the fusion of spleen cells and myeloma cells, and the plating of fused cells in selective HAT medium were all derived from those described by Campbell and Lietzke and Unsicker. method described in detail. Unlike the standard method, our method for producing monoclonal antibodies is to use highly pure NDP-Kinase (7000-fold purity - see Table 1) for the first immunization instead of crude extracts and partially purified enzyme preparations. Ascites fluid was prepared by injecting 3 x 10 6 Myeloma cells were obtained by priming BALB / C mice with 500 l of norphytane one week before. Ascitic fluid was collected after 20 days. IgG immunoglobulins were purified from ascitic fluid by affinity chromatography on a 3 ml Protein ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com