Monoclonal antibody against estrogen-stimulated leucine aminopeptidase
A monoclonal antibody, human technology, applied in the direction of antibody, enzyme, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve the problem of complex sample processing and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0051] Example 1: Isolation and purification of estrogen-dependent LAPase
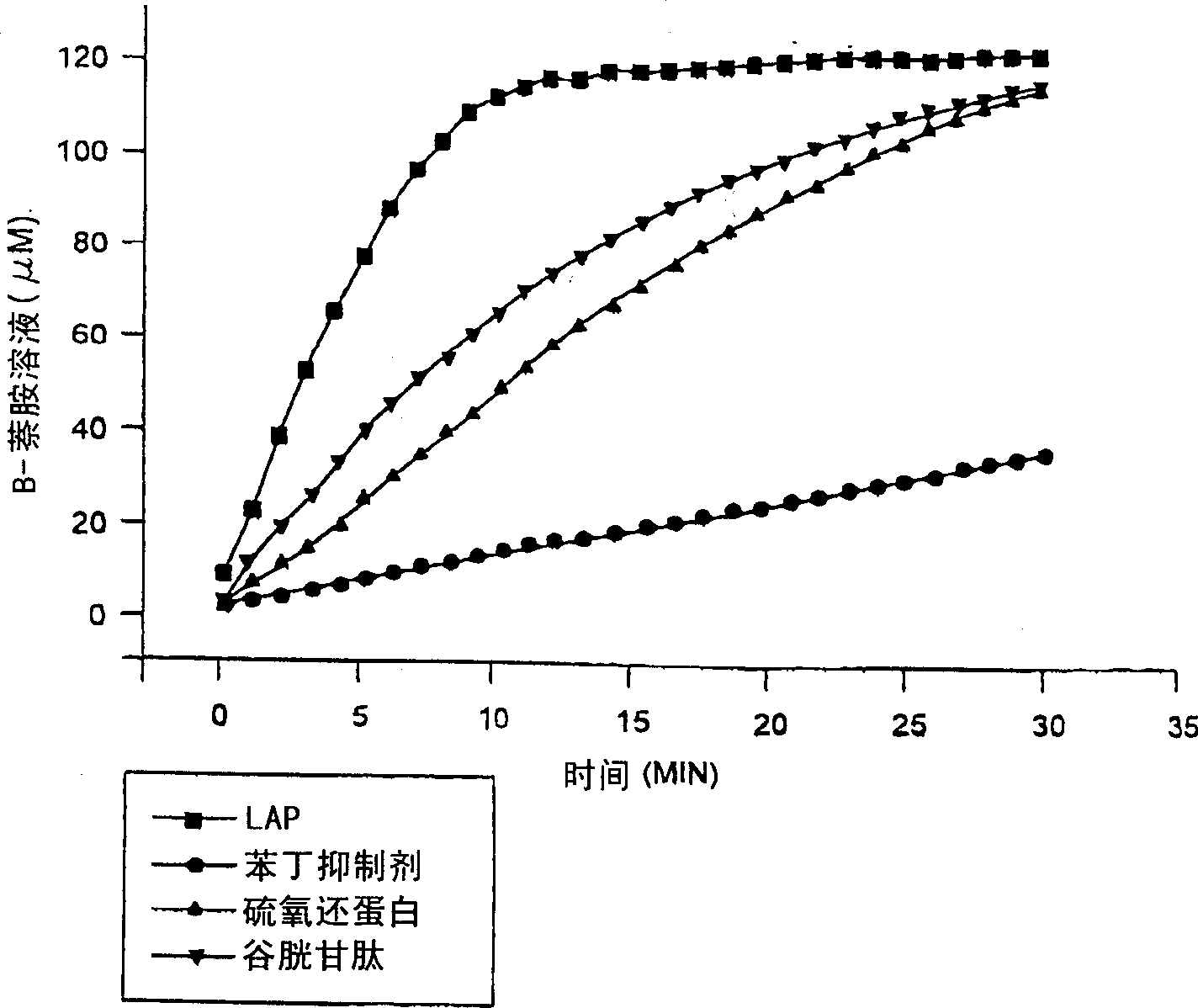
[0052] Breast cancer blasts from human tumor tissue were stimulated with 100nm 17-B-estradiol for 24 hours, or culture medium as control. The cell medium is: RPMI 1640 culture medium + 10% FCS + 100U / ml penicillin + 100ug / ml streptomycin. Supernatants were collected after digestion with PBS in seamless dextran tubes (MV12,400) for 24 hours (4°C). LAP was then purified from the degraded cell supernatant using HPLC-gel permeation chromatography with DEAE-cellulose and Bestatin-Sepharose affinity chromatography. Briefly, the cell supernatant was poured onto a Bio-Sil SEC-250 column (600×7.5 mm) containing 100 mM sodium phosphate (pH 6.8), 100 mM Na 2 SO 4 , 1uM ZnCl 2 , and 10% glycerol buffer pre-equilibrated. The column was then washed with 300ml of the same buffer at a flow rate of 0.5ml / min. The protein was concentrated to 10ml by high-speed centrifugation with YM5 membrane. The concentrated so...
example 2
[0057] A) Production and purification of monoclonal antibodies
[0058] Hybridoma cell line 7B6 producing 17-B-estradiol-stimulated LAPase mouse monoclonal antibody, preparation of immunization antigen, preparation of spleen cells from immunized animals, fusion of spleen cells and myeloma cells, and the selection of fused cells in The coating methods in the media were derived from the method proposed by Campbell and the method described in detail by Lietzhe and Unsicker.
[0059] Briefly, basic immune responses were performed with purified es-LApase after desalting. Boosted with purified es-LApase at days 14, 35, and 56, BALB / C mice were screened at days 24 and 45. Mice were sacrificed on day 59 and the best responding splenocytes were fused with myeloma cells. Screening was performed using dot plot immunostaining on nitrocellulose.
[0060] Hybridoma clone 7B6 was obtained by single cell cloning by limiting dilution. Prepare four serial dilution tubes containing hybridoma...
example 4
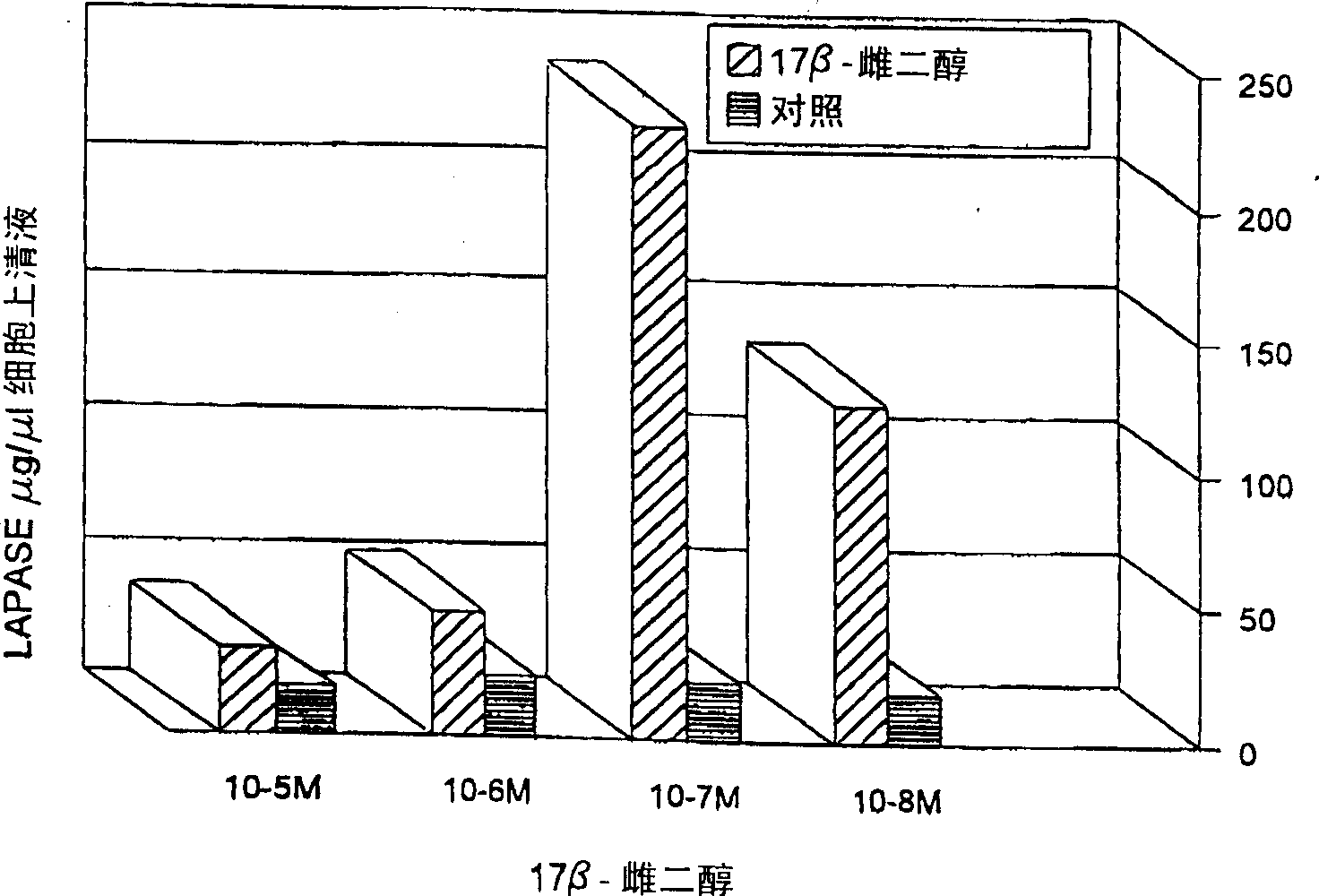
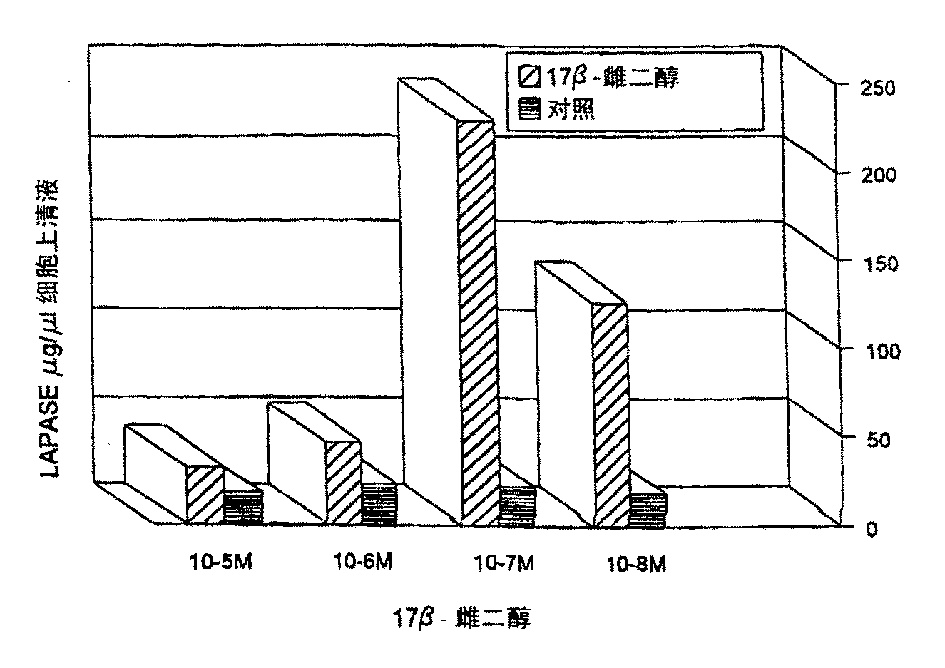
[0084] Example 4: LAPase level in cell supernatant after estrogen stimulation
[0085] Early breast cancer mother cells were cultured with 100nM 17-B-estradiol for 24 hours, and the simple medium was used as blank. Use 7B6 IgGla) - LAPase in the supernatant isolated from protein G-LAPase beads covalently cross-linked to monoclonal antibodies. The activity of LAPase in the supernatant of LAPase immunoprecipitation was determined by fluorescence method using leucine-B-naline as substrate.
[0086] The purified enzyme preparation obtained from Sigma was incubated with 100nM 17-B-estradiol for 24 hours, and the activity of LAPase was measured by fluorescence method using leucine-B-naline as the substrate.
[0087] After estrogen stimulation, the maximum release of LAPase was at an estrogen concentration of 100 nM, after 24 hours of culture (see figure 1 ). During the same period, there was no change in LAPase in the cell supernatant of control cells. In addition, the LAPase ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com