Serial medicine carriers, tyrosine hydrozylase fusion protein as new carrier and its prepn
A fusion protein and carrier technology, applied in the field of peptide chemistry, can solve problems such as toxic side effects and curative effect decline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1 Preparation of PMPV gene vector:
[0013] 1). Preparation of PMPV gene:
[0014] That is, 13 lysine genes were prepared in series—entrusted to Shanghai Sangong Co., Ltd.
[0015] 2). The PMPV gene is combined with the carrier to form the PMPV gene carrier:
[0016] The vector TransVectortm is provided by Q.BIO gene company.
[0017] 1 ug of the vector TransVectortm and PMPV genes were taken, digested with type II restriction enzymes EcoRI and PstI respectively, and then recovered with a plasmid extraction kit (Wizard PCR Preps DNA Purification System). The vector and PMPV gene were mixed at a ratio of 1:3, and 5U of T was added. 4 Ligase, react at 16°C for 16 hours. CaC for reaction product l 2 BL21 bacteria were transformed by heat shock method. The transformed bacteria were plated on LB fixed plate medium containing ampicillin and X-gal for blue-white colony screening. Pick the white colony containing the recombinant and name it BL21PMPV.
[0018] P...
Embodiment 2
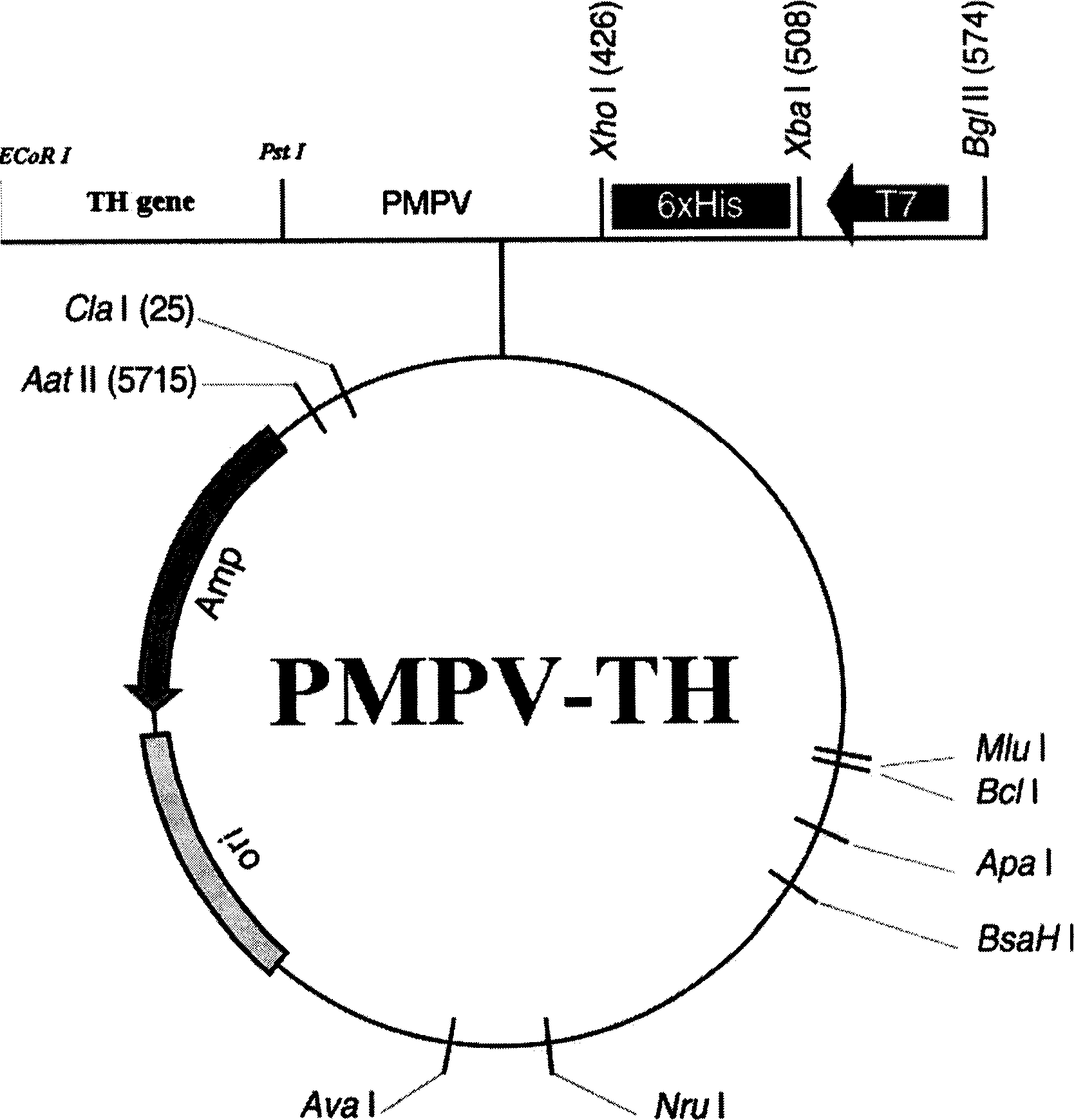
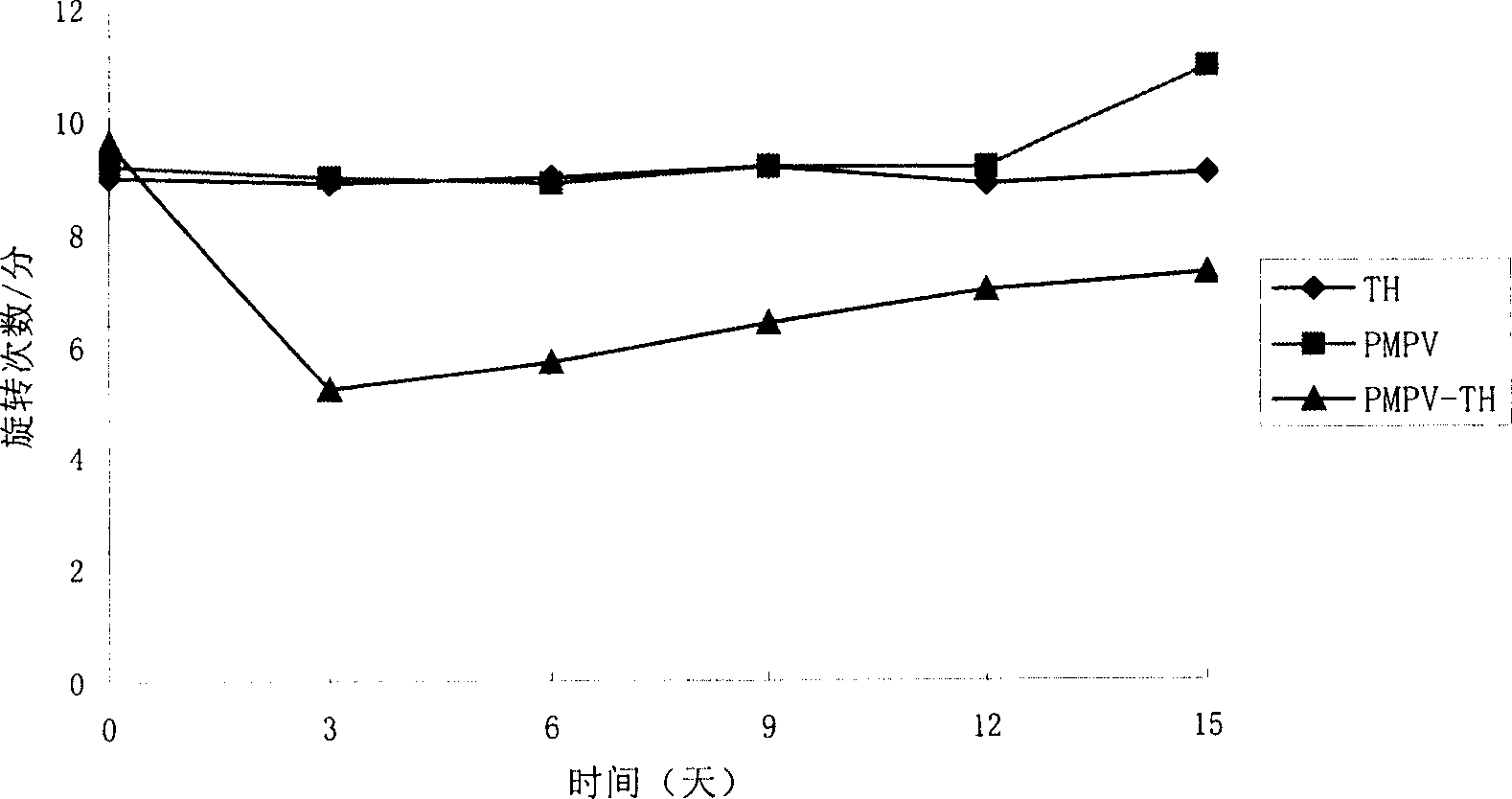
[0019] The total RNAlug of human fetus was taken, and each reaction component was added in sequence according to the operation guide of cDNA synthesis system. The total reaction volume was 20 ul, and after 15 min at 42 °C, it was inactivated at 99 °C for 5 min, and used as a PCR template. The total volume of the PCR reaction system is 50 ul, containing 10 ul of reverse transcription product, and the addition of other components is still carried out according to the above-mentioned operation guide. The first step: start Golden Tap enzyme at 95°C for 12min; the second step: denaturation at 94°C for 50s; the third step: anneal at 54°C for 1min; the fourth step: extend at 72°C for 1.5min; the fifth step: cycle 35 times. PCR amplification bands were checked by 2% agarose gel electrophoresis. Example 3 Preparation of PMPV-TH fusion protein gene vector ( figure 1 ):
Embodiment 3
[0019] The total RNAlug of human fetus was taken, and each reaction component was added in sequence according to the operation guide of cDNA synthesis system. The total reaction volume was 20 ul, and after 15 min at 42 °C, it was inactivated at 99 °C for 5 min, and used as a PCR template. The total volume of the PCR reaction system is 50 ul, containing 10 ul of reverse transcription product, and the addition of other components is still carried out according to the above-mentioned operation guide. The first step: start Golden Tap enzyme at 95°C for 12min; the second step: denaturation at 94°C for 50s; the third step: anneal at 54°C for 1min; the fourth step: extend at 72°C for 1.5min; the fifth step: cycle 35 times. PCR amplification bands were checked by 2% agarose gel electrophoresis. Example 3 Preparation of PMPV-TH fusion protein gene vector ( figure 1 ):
[0020] 1) Take 1ug of each of PMPV vector and TH gene, digest with type II restriction enzymes EcoRI and PstI resp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com