Human interferon alpha 2a gene cDNA code modification recombination sequence
An interferon α, encoding technology, applied in the field of high-efficiency expression of recombinant proteins, can solve the problems of incorrect formation of double disulfide bonds, hindering interferon molecules, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach : Embodiment 1
[0021] The following examples are used to specifically illustrate the embodiments of the present invention: Example 1 Human interferon alpha 2a gene coding modification recombination and high-efficiency expression
[0022] The starting point of this study is to artificially modify and recombine the amino acid code in the cDNA sequence of natural human interferon α2a. According to the published natural human interferon cDNA sequence (Manual et. al. Gene 10: 110, 1980, Streuli et al. Science 209: 1343-1347, 1980, Goeddle et al., Natrue 290: 20-26 1981, GENEBANK) , compared the distribution of amino acid codes in each cDNA sequence, and determined that the artificial modification involved 23 nucleotide residues in the DNA code that coded 20 amino acid residues. See Table 1.
[0023]Table 1: Human interferon α2a gene DNA modification site site coding amino acid original code modified coding 124~126 Pro (proline) CCT CCG163~165 Leu (leucine) CTC CTG178~180 Arg (arginine ) AGG CGC...
Embodiment 2
[0024] The modified recombinant human interferon α2a cDNA sequence has 23 differences from its natural cDNA sequence (Figure 1). However, the amino acid residue sequence of the interferon α2a protein polypeptide encoded by it is the same as the amino acid residue sequence of the natural interferon α2a protein polypeptide. Example 2 Construction of plasmid vectors for cloning and expressing human interferon α2a 1. Construction of expression vectors:
[0025] 1 The original plasmid is pBR322 (4361bp).
[0026] 2 Insert the artificially synthesized DNA fragment (102bp) containing the T-7 promoter, the Lac promoter regulatory sequence and the restriction site sequence for inserting the target gene into the HindIII restriction site at position 29 of pBR322. 3 Use BamH1 and Bsp681 endonucleases to excise part of the TeT gene (about 600bp) contained in pBR322, and insert the Lac gene (1079bp) in this region.
[0027] 4 The reconstructed plasmid is about 5000bp, including the basic ...
Embodiment 3
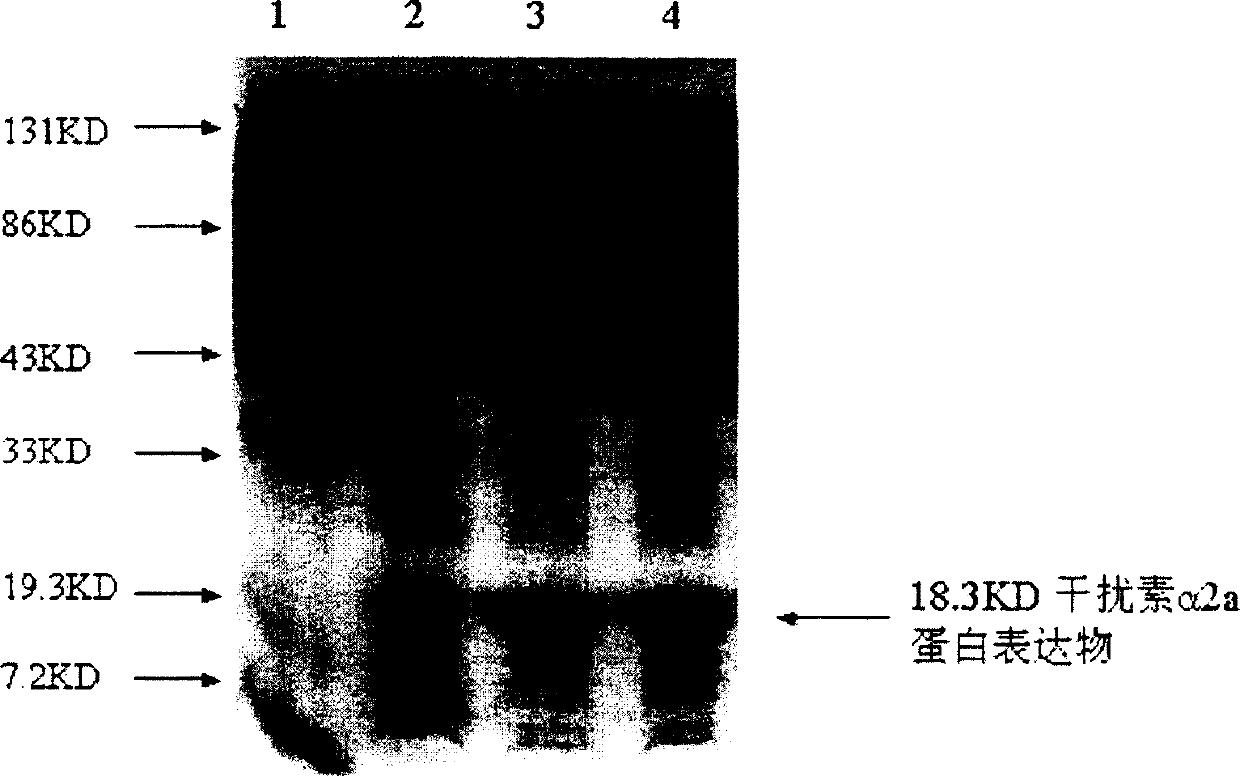
[0032] The N-terminal 18 amino acid sequences of the interferon protein expressed by the human interferon α2a gene after gene recombination and modification were determined, and the result was: 18K-CDLPQ THSLG SRRTL MLL (identification unit MIDWESTANALYTICAL, INC., 11141E SOUTH TOWNE SQUARE St.Louis, MO 63123), and the C-terminus is glutamic acid (E) (identification unit COMMONWEALTH BIOTECHNOLOGIES, INC., 601 BiotechDrive, Richmond, VA 23235), confirming that the amino acid sequence of recombinant modified human interferon α2a is completely consistent with the amino acid sequence of natural interferon α2a. Example 4 Results and protein product activity identification
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com